Lithium batteries - Design - Active materials, electrolytes, and separators for safe design
Jul,30,24
1. Diaphragm protection technology
1.1 Surface Modification
On the basis of the original polyolefin membrane, surface coating can improve the high temperature resistance and electrochemical performance of the membrane.
Coating modified materials mainly include inorganic nanoparticles and organic polymers.
Inorganic modified coating materials include inorganic particles such as Al2O3, SiO2, TiO2, and ZrO2.
Compared with Al2O3, AlOOH ceramic coatings have the advantages of higher heat resistance, lower density,
and lower internal resistance. In the future, AlOOH modified membranes have greater potential for application.
Using boehmite powder with particle sizes of 0.741 μ m and 1.172 μ m as coating materials, PVDF as adhesive, and a 9 μ m thick PP membrane as substrate,
B1 and B2 composite membranes were prepared and their performance was tested. The comprehensive performance of boehmite/PP composite membrane is better than that of PP membrane.
The shrinkage rate of B0 membrane (unmodified PP membrane) exceeds 57% at 140 ℃, while B1 membrane is less than 3% and remains intact at 180 ℃;
The tensile strength of B1 diaphragm is 18.8% higher than that of B0 diaphragm, and the puncture strength of B2 diaphragm is 54.4% higher than that of B0 diaphragm;
Within 30 seconds, the electrolyte can completely infiltrate the B2 diaphragm, while the infiltration area of the B0 diaphragm is less than 1/2.
Al2O3、 Nano inorganic coatings such as boehmite can increase the heat resistance of membranes, but they are also prone to clogging the membrane pores and hindering the transport of Li+.
Therefore, researchers have used high polymers as coating materials to modify polyolefin membranes.
This type of polymer includes PVDF, PVDC, ANF, PAN, PMMA, PDA, etc.
Coating polyolefin membranes with PVDF and copolymers is currently a relatively mature method of membrane modification.
1.2 Different diaphragm systems
Polyimide (PI) based separators are considered the next generation of lithium-ion battery separator materials due to their excellent heat resistance, chemical stability, and ideal mechanical properties.
The PI separator prepared by electrospinning method has the advantages of low cost, high controllability, and high porosity.
However, the mechanical strength of the prepared separator is poor, and the pore size is large and the pore size distribution is wide, which may exacerbate battery self discharge and crosstalk reactions.
In addition, electrospinning still faces many bottlenecks in industrial scale manufacturing due to low productivity, poor reproducibility, and environmental pollution issues.
In this regard, Y. R. Deng et al. prepared a PI aerogel (PIA) diaphragm with uniform porosity, high temperature resistance and good electrochemical performance by using the sol gel method and supercritical drying,
and applied it in lithium ion batteries. The porosity (78.35%) and electrolyte absorption rate (321.66%) of PIA separator are relatively high, which helps to improve the electrochemical performance of lithium-ion batteries.
The LiFePO4 Li half cell with PIA separator can stably cycle for more than 1000 times at a rate of 1C and 2.8-4.2V, with a capacity retention rate of over 80%.
Thanks to the high thermal stability of PIA, LiFePO4 Li half cells using PIA separators can cycle stably at 120 ℃.
To determine the improvement effect on the safety performance of lithium-ion batteries, a flexible packaging battery was assembled using LiFePO4 positive electrode, PIA separator, and graphite negative electrode.
Compared with Celgard 2400 separator, the thermal runaway behavior of the entire battery was studied using an accelerated calorimeter (ARC).
It was found that the thermal runaway temperature of the battery using PIA separator could be increased from 131 ℃ using Celgard 2400 separator to 170 ℃, an increase of about 30%.
Among numerous system membranes, there are also polyethylene terephthalate (PET), cellulose, and fluoropolymer membranes.
The main performance parameters comparison between several types of membranes and polyolefin (PP or PE) membranes is shown in Table 1.
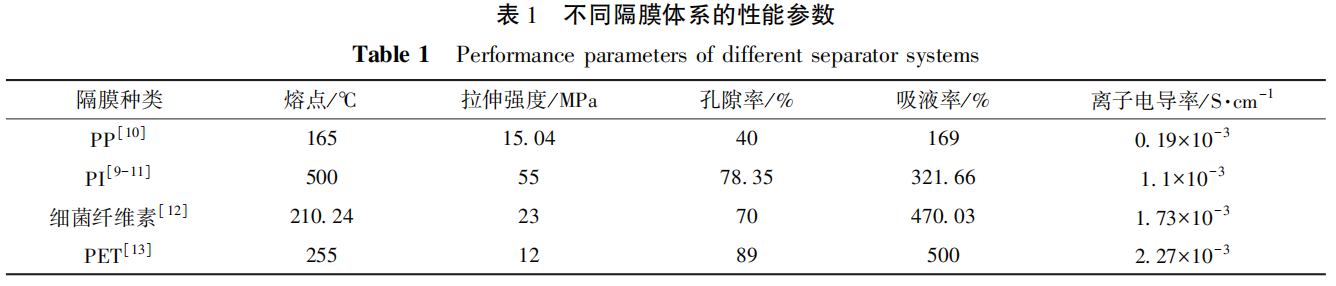
From Table 1, it can be seen that these separators have significantly improved thermal stability and liquid absorption rate, providing more choices for the development of high safety lithium-ion batteries.
1.3 Thermal sealing diaphragm
A heat sealed diaphragm is a type of diaphragm that closes pores and blocks ion channels at a certain temperature.
The initial heat sealed membrane was coated with paraffin microspheres on the surface of PP membrane, but due to the large particle size and uneven coating of the microspheres,
the rate performance of the battery was affected to some extent. In addition, paraffin microspheres respond slowly when the temperature rises rapidly,
which can easily cause a temperature response lag and fail to suppress the thermal runaway behavior of the battery.
Therefore, W. X. Ji et al. proposed a heat sealed membrane modified with ethylene vinyl acetate copolymer microspheres.
Thanks to the suitable thermal response temperature (90 ℃), small particle size (about 1 μ m),
and high chemical and electrochemical stability of ethylene vinyl acetate copolymer microspheres,
microsphere modified membranes can ensure both electrochemical performance and reliable high-temperature thermal shutdown function.
Assemble 20Ah lithium cobalt oxide graphite flexible packaging batteries using PP separators and modified separators respectively for short-circuit testing.
The results showed that at the beginning of the short circuit, the voltage of the battery using PP separator dropped sharply,
generating a large short-circuit current and releasing a large amount of Joule heat, causing the internal temperature of the battery to quickly reach 131.2 ℃,
until the voltage dropped to 0V and the temperature began to decrease;
The flexible packaging battery using ethylene vinyl acetate copolymer microspheres coated with separators, at the moment of external short circuit,
the open circuit voltage suddenly drops and then suddenly rises again.
Throughout the process, the highest surface temperature of the battery cell is only 57.2 ℃.
This is because the Joule heating caused by external short circuit causes the melting and collapse of the copolymer microsphere layer coated on the surface of the separator,
which transforms into a dense polymer insulation layer on the surface of the PP separator.
This breaks the Li+transfer between the positive and negative electrodes inside the battery, putting the battery in a disconnected state.
From this, it can be seen that heat sealed separators can prevent severe temperature rise of batteries in the event of external short circuits,
improve the safety of large capacity lithium-ion batteries, and demonstrate good application prospects.
1.4 Heat absorbing diaphragm
Z. F. Liu et al. prepared a phase change temperature regulating membrane that can absorb the heat generated inside the battery in situ.
Integrating phase change material (PCM) with thermal storage function into PAN fiber membrane, endowing the membrane with temperature regulation function. Under abusive conditions,
the internal PCM is heated and melted, accompanied by a large amount of latent heat storage, which can timely absorb the heat generated inside the battery and prevent thermal runaway.
Under normal working conditions, due to the high porosity and good electrolyte affinity of the PAN fiber membrane,
batteries assembled based on this membrane material exhibit low polarization potential, rapid ion transport, and other characteristics, demonstrating ideal electrochemical performance.
The 63mAh lithium iron phosphate graphite lithium-ion battery assembled based on this type of separator material can recover to room temperature within 35 seconds after needle puncture experiment.
This indicates that the phase change temperature regulating diaphragm has good temperature regulating ability for batteries after internal short circuit,
providing internal overheating protection for high-energy density lithium-ion batteries and a method to improve the safety of lithium-ion batteries.
The needle puncture experiment was conducted based on a 63mAh lithium iron phosphate graphite lithium-ion battery, which has a relatively small capacity.
Its temperature regulation ability and practical prospects in high-capacity batteries still need to be verified.
2. Safe electrolyte
2.1 Ionic liquids
Ionic liquid is a molten salt with a melting point below 100 ℃, composed only of cations and anions in the molten state.
The high number of ions in ionic liquids endows them with high conductivity, as well as excellent thermal stability, chemical stability, electrochemical redox stability, low volatility,
and low reaction heat with active electrode materials. More importantly, they are completely non flammable, making them a highly safe electrolyte.
The complete absence of solvent molecules in the electrolyte can lead to a series of problems, such as the inability of most ionic liquids to decompose and form stable SEI films,
and poor compatibility with carbon based materials such as graphite anodes. Therefore, only higher cost Li4Ti5O12 or non carbon anodes can be used.
The introduction of film-forming additives or lithium difluorosulfonyl imide (LiFSI), as well as the use of high concentration salt electrolytes, can improve interfacial stability,
but they still cannot solve the problem of poor rate performance of electrode materials caused by the high viscosity, poor wettability, and low Li+diffusion coefficient of ionic liquids.
Carbonate solvents have low viscosity and high dielectric constant, which can improve the physical and chemical properties of ionic liquids and decompose to form stable SEI films.
Mixing ionic liquids with carbonate solvents to prepare non combustible electrolytes is a method to balance the rate performance and safety of batteries.
The improvement effect of viscosity, wettability, and Li+diffusion coefficient of the electrolyte after blending is limited;
Moreover, the electrolyte contains 20% flammable compounds, which still pose certain safety hazards to lithium-ion batteries.
Mixing high flash point, non flammable sulfone or fluorinated ether solvents with ionic liquids can further enhance the safety of batteries.
2.2 Fluorinated solvents
Fluorinated solvents are a type of lithium-ion battery electrolyte solvent that has been extensively studied and widely used in high safety lithium-ion battery electrolytes.
Fluorine atoms have smaller atomic radii, stronger electronegativity, lower polarizability, and fluorinated solvents have advantages such as low freezing point, high flash point,
and good wettability with electrodes.
Some fluorinated ethers with high degrees of fluorination have flame-retardant or non flammable properties and can be used as solvents to improve the safety of electrolytes.
Due to the electron withdrawing effect of fluorine atoms, the
highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels of fluorinated solvents decrease,
the antioxidant stability increases, and it is easier to reduce on the negative electrode surface,
forming an SEI film rich in inorganic substances and improving the electrochemical compatibility between the electrolyte and electrode materials.
Fluorinated alkyl ethers are a type of fluorinated solvent that has been extensively studied.
Among them, perfluoroalkyl ethers have significant flame retardant or non flammable effects, but have poor miscibility with other solvents, weak solubility for lithium salts, and high cost.
They can only be used as additives (<10%). Usually, partially fluorinated or semi alkyl perfluorinated ethers are used as co solvents (20%~50%), but some flame retardant properties may be lost.
Although fluorinated solvents can serve as co solvents to replace some highly volatile and flammable solvents, the electrolyte is completely non flammable only when the volume fraction is high (>70%).
There were early reports of using methyl nonafluoron-butyl ether as a flame retardant solvent, but its application in the lithium-ion battery industry was very limited.
The widely used product is 1,1,2,2-tetrafluoroethyl-2,2,3,3-tetrafluoropropyl ether, with the trade name D2. D2 has an appropriate boiling point and good oxidation resistance,
and is also a high-voltage additive.
X. L. Fan et al. developed a high voltage resistant, non flammable perfluorinated electrolyte by combining fluorinated ether D2, trifluoroethylmethyl carbonate (FEMC),
and fluorinated vinyl carbonate (FEC),
which has been applied to high nickel ternary cathode materials and 5V cobalt lithium phosphate metal batteries with good performance.
2.3 Organic phosphate ester solvents
Organic phosphate compounds have the characteristics of high boiling point, low viscosity, and high dielectric constant.
Compared to ionic liquids and fluorinated ethers. This type of compound has the characteristics of low cost and easy synthesis.
At the same time. It has a similar molecular structure to carbonates. It is a solvent that has the potential to achieve flame retardant/non flammable electrolytes.
At present, almost all phosphate ester solvents reported in literature are incompatible with graphite negative electrodes,
meaning that graphite cannot undergo reversible lithium insertion and extraction reactions stably and efficiently in existing phosphate ester based electrolytes.
The primary task in developing phosphate ester electrolytes is to address the compatibility issue between organic phosphate ester solvents and graphite.
The development of existing organic phosphate ester solvents mainly includes phosphate ester, hypophosphite ester, and phosphonate ester solvents.
As mentioned earlier, organic phosphate ester solvents are not compatible with graphite negative electrodes.
During charging and discharging, a stable SEI film cannot be formed on the negative electrode surface, and it can also lead to co embedding and damage to the layered structure of graphite.
Therefore, in early research on organic phosphate esters, they were only used as flame retardant additives or co solvents added to electrolytes to reduce their flammability.
Experiments have shown that when the concentration of organic phosphate esters added to the electrolyte is too low (<10%),
there is no significant flame retardant effect; However, when the concentration is high (>20%), it will also inhibit the lithium insertion ability of graphite negative electrodes.
2.4 Phosphoronitrile flame retardants
Phosphoronitrile compounds are a type of composite flame retardant additive.
It mainly includes polymer linear phosphorus nitrogen compounds and small molecule cyclic phosphorus nitrogen compounds.
The main characteristics of phosphazene flame retardants are. A small amount of addition (with a mass fraction of 5%~15%) can achieve the flame retardant or non combustible effect of the electrolyte.
And it has good compatibility with electrode materials. The impact on the electrochemical performance of lithium-ion batteries is minimal.
Bridgestone's cyclic phosphazene (PFPN) is an early invented flame retardant with a high electrochemical oxidation window.
It has been widely used in high-voltage lithium-ion batteries, such as those using high-voltage lithium cobalt oxide cathode materials or 5V high-voltage lithium nickel manganese oxide materials.
3. Positive electrode coating technology
Surface coating can improve the thermal stability of positive electrode materials and is currently the main positive electrode protection technology.
Coating other highly stable materials on the surface of the positive electrode material can prevent direct contact between the positive electrode material and the electrolyte,
thereby suppressing the phase transition of the positive electrode material, improving thermal stability, and reducing the disorder of cations on lattice sites.
This type of coating should have good thermal stability and chemical inertness, and the coating materials mainly include phosphates, fluorides, and solid oxides.
Phosphates with strong PO4 covalent bonds coated on the surface of the positive electrode material can enhance its thermal stability.
If the positive electrode is coated with AlPO4, it has good thermal stability and exhibits good performance in overcharge tests.
M. Yoon et al. reported a room temperature coating synthesis strategy of "coating+perfusion",
which applied cobalt boride (CoB) metallic glass to nickel rich layered cathode material NCM811,
achieving full surface coverage of secondary particles and grain boundary wetting of the cathode material, improving rate performance and cycling stability.
After 500 cycles at 2.8~4.3V at 1C, the capacity retention rate of the material increased from 79.2% before coating to 95.0%.
Research has shown that ideal sexual energy can simultaneously suppress microstructural degradation and side reactions with interfaces.
M. Jo et al. achieved uniform coating of Mn3 (PO4) 2 nanocrystals on the surface of NCM622 cathode by sol-gel method at a lower temperature.
The Mn3 (PO4) 2 coating reduces the direct contact between the electrolyte and the unstable oxidation positive electrode, thereby reducing the degree of exothermic side reactions.
4 Negative electrode modification strategies
Graphite itself is relatively stable, but lithium embedded graphite will continue to react with the electrolyte at high temperatures,
exacerbating the initial heat accumulation of thermal runaway and promoting the chain reaction of thermal runaway.
The SEI film can isolate the direct contact between the negative electrode and the electrolyte, improving the stability of the negative electrode.
Therefore, constructing a high thermal stability SEI film is a key method to isolate the negative electrode from the electrolyte side reaction and suppress thermal runaway.
Introducing film-forming additives into the electrolyte can improve the structure and properties of the SEI film.
Ammonium perfluorooctanoate (APC), ethylene carbonate (VC), and ethylene carbonate (VEC) can preferentially reduce and decompose the electrolyte,
forming a uniform and dense polymer film on the surface of the graphite negative electrode, enhancing the thermal stability of the SEI film.
Starting from surface modification of materials,
the thermal stability of negative electrode materials can be improved by constructing artificial SEI films such as metal and metal oxide deposition layers, polymer or carbon coating layers.
As the temperature rises, the SEI film constructed by the above two methods will always decompose.
At higher temperatures, the exothermic reaction between the lithium graphite negative electrode and the electrolyte will be more intense.
In addition, during high current charging, the lithium evolution reaction of the graphite negative electrode can also pose a risk of thermal runaway in lithium-ion batteries.
The charging current rate determines the Li+flux per unit area of negative electrode material.
When the solid-phase diffusion process of Li+in the negative electrode is slow (such as when the temperature is too low or the state of charge is high),
and the charging current density is too high, lithium precipitation reaction will occur on the surface of the negative electrode,
and the precipitated lithium dendrites will puncture the separator, causing internal short circuit and catastrophic consequences such as combustion and explosion.
Shortening the diffusion path of Li+between graphite layers and increasing the interlayer spacing of graphite can accelerate the solid-phase diffusion of Li+between graphite layers.
5 Conclusion and Prospect
Lithium ion battery technology is mature, suitable for large-scale application and mass production,
and is currently the key development direction for electric vehicles and large-scale energy storage technology.
The energy density of lithium-ion batteries is constantly increasing, and there is a greater demand for battery safety.
Therefore, safety is an important indicator for the development of lithium-ion batteries.
The author of this article systematically summarizes the existing methods for blocking thermal runaway and improving the safety of lithium-ion batteries from the perspectives of separators, electrolytes,
and electrode materials.
By summarizing the current research on improving the safety of lithium-ion batteries and combining it with the new mechanism of thermal runaway,
several key directions for the development of safety materials for lithium-ion batteries in the future are proposed
① The use of inorganic nanoparticles for surface modification of polyolefin membranes can improve their thermal stability, but the improvement effect is limited.
Separators with high thermal stability and mechanical strength will provide more choices for high safety lithium-ion batteries.
In addition, intelligent thermal responsive membranes can be designed,
such as thermal sealing membranes that can cut off ion transport at high temperatures, fire-resistant membranes that release flame retardants, and phase change heat absorbing membranes.
The above safety diaphragm design strategies are all based on the thermal runaway caused by diaphragm melting,
but internal short circuit is not the only factor triggering thermal runaway in lithium-ion batteries.
At high temperatures, the intense oxidation-reduction reaction between the active oxygen released by the positive electrode and the electrolyte,
as well as the lithium graphite negative electrode, is also the main reason for the runaway of contact heat.
How to block the cross-talk reaction of active oxygen released from the positive electrode while ensuring the high temperature resistance of the separator is an important measure for the future development of safety separators.
② Commercialized lithium-ion battery electrolytes generally have low flash points and are prone to combustion or even explosion at high temperatures.
Developing flame-retardant/non combustible electrolytes to reduce their flammability is one of the measures to improve the safety of lithium-ion batteries.
Based on this method, extensive research has been conducted on flame retardant/non combustible electrolytes,
mainly including ionic liquids, fluorinated solvents, organic phosphate ester solvents, phosphazene flame retardants,
and high concentration salt electrolytes. Based on the temporal characteristics of thermal runaway,
the combustion of electrolyte is the main energy source in the later stage of thermal runaway,
while the exothermic side reaction between electrolyte and lithium graphite after SEI film rupture in the early stage helps to accumulate heat in the early stage of thermal runaway.
Starting from the electrolyte, real-time targeted repair of damaged SEI film. Inhibit the reaction between lithiated graphite and electrolyte. It will be a strategy to suppress thermal runaway.
③ Direct contact between the positive electrode material and electrolyte at high temperatures can cause irreversible phase transitions on the surface of the positive electrode material.
Reduce the thermal stability of the material. The design of safe positive electrode materials mainly focuses on isolating direct contact between active positive electrode materials and electrolytes,
including surface coating of positive electrode materials and the use of single-crystal ternary positive electrode materials without lattice gaps.
In addition to the design strategies for safe positive electrode materials for lithium-ion batteries summarized by the author of this article,
active oxygen capture coatings can also be developed to quench the active oxygen released from the thermal decomposition of positive electrode materials such as ternary,
lithium cobalt oxide, and lithium manganese oxide, thereby avoiding the reaction of active oxygen with electrolytes or lithium graphite negative electrodes.
④ The reaction activity between exposed lithium embedded graphite and electrolyte is high,
and the traditional improvement strategy is to add film-forming additives to the electrolyte or construct an artificial SEI film.
The failure of SEI film under high temperature will ultimately lead to the reaction between lithium embedded graphite and electrolyte.
Therefore, it is necessary to develop a real-time in-situ repair technology for SEI film to block the reaction between lithium graphite and electrolyte.






