Production process of positive electrode material for ternary lithium batteries
Aug,01,24
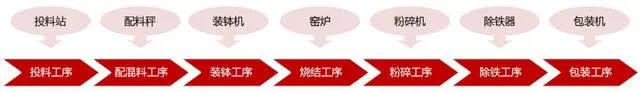
The production process of positive electrode materials for ternary lithium batteries mainly includes steps such as batching, mixing, sintering, crushing, screening, and packaging.
Ingredients: Accurately weigh various raw materials, such as compounds of nickel, cobalt, manganese, or aluminum,
according to the required proportion of the ternary positive electrode material formula, and mix them evenly.
Mixing: Put the weighed raw materials into the mixer for thorough mixing,
ensuring that various raw materials can be evenly dispersed and prepared for the subsequent high-temperature sintering process.
Sintering: Put the uniformly mixed raw materials into a high-temperature furnace for sintering.
At high temperatures, chemical reactions occur between raw materials to synthesize ternary positive electrode materials.
The temperature and time of sintering depend on the specific material formula and the required battery performance.
Crushing and sieving: After sintering is completed, the product usually needs to be crushed to obtain the desired particle size powder.
Subsequently, oversized or undersized particles are removed through screening to ensure that the particle size distribution of the ternary positive electrode material meets the requirements.
Surface treatment: In order to improve the electrochemical performance of ternary cathode materials,
it is sometimes necessary to perform surface treatment on the powder, such as coating or doping with other elements.
Packaging and Storage: Finally, package the processed ternary positive electrode material to ensure its quality and stability.
Packaging is usually done by vacuum packaging or by filling inert gas after vacuum to prevent the material from getting damp or reacting with oxygen in the air.
The control of parameters such as temperature, pressure, and time is crucial throughout the entire production process, as they directly affect the performance and quality of the final product.
In addition, strict quality control and testing are required during the production process to ensure product quality and consistency.
It is worth noting that in addition to the main process steps mentioned above, some auxiliary steps may also be included, such as grading, vibrating screen, etc.,
to further improve the particle size distribution and purity of the product.
Meanwhile, the production process may be adjusted and optimized based on specific materials and requirements.
1、 Common processes for positive electrode materials: high-temperature solid-state reaction
In the preparation of inorganic electrode materials for lithium-ion batteries, the most commonly used method is high-temperature solid-state reaction.
High temperature solid-phase reaction: refers to the process in which reactants, including solid-phase substances,
react at a certain temperature for a period of time,
undergo chemical reactions through the mutual diffusion of various elements, and generate the most stable compounds at a certain temperature.
This includes solid-state reactions, solid gas phase reactions, and solid-liquid phase reactions.
① Even if the sol gel method, coprecipitation method, hydrothermal method and solvothermal method are used,
the solid phase reaction or solid phase sintering is usually required at a higher temperature.
This is because the working principle of lithium-ion batteries requires their electrode materials to repeatedly embed and remove Li+,
so their lattice structure must have sufficient stability, which requires high crystallinity and regular crystal structure of the active material.
This is difficult to achieve under low temperature conditions, so the electrode materials currently used in lithium-ion batteries are mostly obtained through high-temperature solid-state reactions.
The positive electrode material processing production line mainly includes mixing system, sintering system,
crushing system, water washing system (only high nickel), packaging system, powder conveying system,
and intelligent control system.
2、 Introduction to the specific process flow of different positive electrode materials
2.1 Introduction to the specific process flow of lithium iron phosphate cathode material
Lithium iron phosphate is relatively inexpensive, environmentally friendly, has high safety performance,
and good high-temperature performance, making it widely used in the market.
However, its energy density is low and its low-temperature performance is poor.
Currently, it is mainly used in the field of commercial vehicles (buses),
and its application in downstream passenger car power batteries is not as good as ternary positive electrode materials with higher energy density.
With the promotion of BYD blade batteries, the application market of lithium iron phosphate may open up.
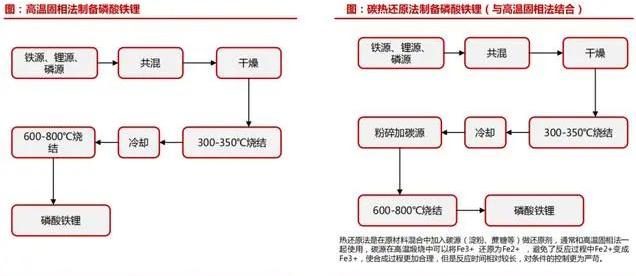
The preparation of lithium iron phosphate powder will to some extent affect its performance as a positive electrode material.
At present, there are many methods to prepare lithium iron phosphate,
such as high temperature solid state reaction method, carbothermal reduction method, hydrothermal method, spray pyrolysis method, sol gel method, coprecipitation method, etc.
At present, the investment in equipment for lithium iron phosphate production lines (excluding precursors) is approximately 100 million yuan/10000 tons (the investment amount varies among different manufacturers).
2.2. Introduction to the specific process flow of ternary positive electrode materials
The positive electrode material adopts a ternary polymer containing three metal elements:
nickel, cobalt, and manganese (or aluminum), among which the nickel cobalt manganese ternary NCM and the nickel cobalt aluminum ternary NCA are used.
The advantages of ternary materials lie in their high energy density, relatively low cost, and excellent low cycle performance,
making them the most promising type of cathode material currently in mass production.
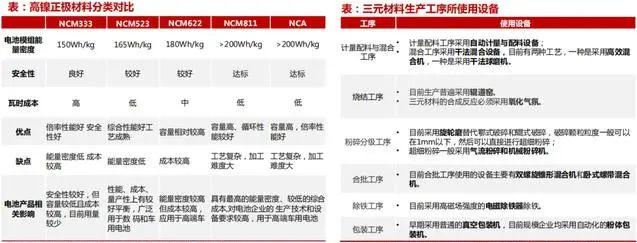
The trend of NCM ternary development is high nickel.
The ternary positive electrode material mainly increases the energy density by increasing the nickel content, charging voltage upper limit, and compaction density.
High nickel positive electrode usually refers to material models with a nickel relative content of 0.6 (inclusive) or more.
Therefore, in terms of energy density, NCA>NCM811>NCM622>NCM523>NCM333。
The production of ternary materials uses nickel cobalt manganese hydroxide, lithium carbonate, and other doping elements as raw materials,
and carries out processes such as batching, mixing, sintering, crushing, batching, screening, iron removal, and packaging.
At present, the investment in equipment for ternary material production lines (excluding precursors) is approximately 150-200 million yuan/10000 tons (with a larger investment in high nickel ternary materials).
High nickel NCM has higher production requirements than conventional NCM materials:
Due to its strong oxidizing properties, high nickel ternary cathode materials require doping and coating for product modification before use.
The selection and distribution uniformity of doping and coating elements depend on the manufacturer's technical processes and production equipment.
Raw materials: For conventional ternary cathode materials, lithium carbonate is generally used as the lithium source material.
High nickel ternary materials require higher energy density and better charge discharge performance, and lithium hydroxide is commonly used as the lithium source material.
Production equipment: High nickel ternary materials are particularly prone to the problem of metal ion mixing (different metal ion mixing occupies space,
which has adverse effects on the electrochemical properties such as initial efficiency, reversible capacity, and cycling performance of the material), and need to be eliminated as much as possible.
Therefore, production needs to be carried out in a pure oxygen environment.
Therefore, the sintering of high nickel products requires an oxygen furnace, while conventional ternary materials only need to use an air furnace.
Production environment: High nickel ternary materials have higher humidity requirements and generally require specialized dehumidification and ventilation equipment. In terms of magnetic material control, high nickel ternary materials also have higher requirements, often requiring specific modifications to factory facilities.
3. Introduction to the specific process flow of lithium cobalt oxide cathode material
Lithium cobalt oxide, also known as lithium cobalt oxide or lithium cobalt oxide,
has become the earliest positive electrode material used in commercial lithium-ion batteries due to its simple synthesis method,
long cycle life, high operating voltage, and good rate performance.
It is widely used in small rechargeable batteries.
Lithium cobalt oxide cathode material has high cost (expensive metal cobalt), poor cycling performance,
and poor safety performance, and has gradually been replaced by ternary cathode materials in recent years.
In the field of ultra-thin electronic products, lithium cobalt oxide cathode materials cannot be replaced due to their advantages such as good volumetric energy density and rate performance.
The production of lithium cobalt oxide uses cobalt oxide, lithium carbonate, and other doping elements as raw materials,
and undergoes processes such as metering, batching, mixing, sintering, crushing and grading, iron removal, and packaging.
At present, the investment amount of lithium cobalt oxide production line equipment (excluding precursors) is approximately 170 million yuan/10000 tons (the investment amount varies among different manufacturers).

2.4 Introduction to the specific process flow of lithium manganese oxide cathode material
Lithium manganese oxide is the earliest researched positive electrode material for lithium batteries, apart from lithium cobalt oxide.
It has the advantages of abundant resources, low cost, and good safety performance;
However, its low specific capacity and poor cycling performance, especially its high-temperature cycling performance, have greatly limited its application.
Lithium manganese oxide batteries will mainly have a certain market share in logistics vehicles and micro passenger vehicles that focus on cost and have relatively low requirements for range.
At present, the most common method for large-scale production of lithium-ion battery cathode material lithium manganese oxide is the high-temperature solid-phase method,
which involves uniformly mixing two solid raw materials containing lithium and manganese separately and calcining them at a certain temperature and time to produce the material.
At present, lithium manganese oxide production lines are relatively low-end, and equipment investment (excluding precursors) is relatively low.
Ternary materials include nickel cobalt manganese (NCM) and nickel cobalt aluminum (NCA), with NCM as an example.
A、 Ternary materials: The different ratios of three elements in ternary materials result in different properties of ternary positive electrode materials, meeting diverse application needs.
Ternary materials combine the advantages of lithium cobalt oxide, lithium nickel oxide, and lithium manganese oxide, and exhibit significant ternary synergistic effects.
Compared to positive electrode materials such as lithium iron phosphate and lithium manganese oxide, ternary materials have higher energy density and longer range.
B、 Lithium cobalt oxide: As the first generation commercialized positive electrode material for lithium batteries,
lithium cobalt oxide has good electrochemical and processing properties, as well as relatively high specific capacity,
and is widely used in small rechargeable batteries. Especially in the field of mid to high end ultra-thin electronic products,
its advantages such as good volumetric energy density and rate performance are obvious.
C、 Lithium manganese oxide: Lithium manganese oxide is the earliest studied positive electrode material for lithium batteries after lithium cobalt oxide.
Compared with lithium cobalt oxide, it has the advantages of abundant resources, low cost, no pollution, good safety performance, and good rate performance;
However, its low specific capacity and poor cycling performance, especially its high-temperature cycling performance, have greatly limited its application.
Lithium manganese oxide batteries mainly have a certain market share in logistics vehicles and micro passenger vehicles that focus on cost and have relatively low requirements for range.
D、 Lithium iron phosphate: Its low price, environmental friendliness, high safety, good structural stability, and cycling performance have made it widely used in the market.
However, its energy density is low and its low-temperature performance is poor. Currently, it is mainly used in commercial vehicles (buses) and energy storage fields.
Preparation process of ternary materials
1. High temperature solid-phase method
The high-temperature solid-phase method is the most commonly used method for preparing metal oxides.
The so-called solid-phase method generally refers to the method of obtaining the desired sample by uniformly mixing solid compounds as raw materials in a stoichiometric ratio,
and then calcining them for a period of time under a certain atmosphere and temperature.
Among them, factors such as the uniformity of raw material mixing, roasting time, heating and cooling rate, atmosphere,
and temperature stability determine the microstructure and electrochemical properties of the product.
Solid phase synthesis materials have the characteristics of low equipment requirements, simple process, easy operation, mature technology, low cost, and large output.
The biggest disadvantage of using this method is the difficulty in controlling material quality, including particle morphology, composition, and size, which leads to poor consistency, stability,
and reproducibility of electrochemical performance.
Xu et al. used Ni (OH) 2 and Li2CO3 as raw materials, with an excess lithium content of 10%, and sintered LiNiO2 at 750 ℃ in an oxygen atmosphere for 12 hours.
A button half cell was prepared using active materials, small particle conductive carbon black, and polyvinylidene fluoride in a mass ratio of 8:1:1.
The test voltage range was between 2.7-4.3 V, and the first discharge specific capacity was 205 mAh/g at 0.1 C.
The first discharge efficiency was 84.7%, and it exhibited good cycling performance.
2. Co precipitation method
Co precipitation method, also known as liquid-phase method, is based on precipitation reaction.
Generally, a salt solution of one or more metal ions is used as the raw material,
and a precipitate is produced through a co current reaction under the action of a precipitant and a complexing agent.
After filtration, washing, and drying processes, the product or precursor is obtained,
which is mixed with lithium salt in solid phase and calcined at a certain temperature and atmosphere for a certain period of time to obtain the positive electrode material.
The co precipitation method has the advantages of accurate reaction measurement, low reaction temperature, simple operation, easy control of conditions,
good reproducibility, and stable electrochemical performance, and has become the main method for synthesizing this material for commercial use.
However, during the co precipitation process, the concentration of reactants, temperature, pH value, feeding rate,
and stirring rate determine the physical parameters such as particle size, element distribution, and crystal form of the material.
Therefore, it is necessary to strictly control various process parameters.
Li et al. prepared spherical layered oxide LiNi0.5Co0.2Mn0.3O2 using high-temperature solid-state reaction. Using Ni0.5Co0.2Mn0.3 (OH) 2 and Li2CO3 as raw materials,
with a lithium content of 1.06. The sintering treatment scheme involves pre sintering at 500 ℃ for 5 hours, followed by sintering at 960 ℃ for 12 hours in an air atmosphere to obtain the sample.
Hua et al. prepared a layered precursor of Ni1/3Co1/3Mn1/3 (OH) 2 using a mixed hydroxide co precipitation method.
The influence of pH control during the reaction process on particle structure, morphology, particle size distribution, and tap density was investigated.
The split (006, 102) and (108, 110) characteristic peaks in the X-ray diffraction spectroscopy test results indicate that the material has a layered structure.
The transmission electron microscopy test results indicate that the (001) crystal plane is preferentially oriented during the co precipitation reaction process.
The electrochemical performance test results indicate that the layered structure of the precursor determines the electrochemical performance of LiNi1/3Co1/3Mn1/3O2.
3 Sol gel method
The sol gel method uses metal organic salt or inorganic salt solution as raw material, and hydrolysis,
condensation and other reactions occur under the action of complexing agent to form a metastable sol system.
Under the action of aging and other conditions, the sol forms a gel with relatively fixed solid particle position.
The gel is dried to get a dry gel with developed spatial structure and uniform distribution of metal ions.
The residual organic impurities in the dry gel system are removed by heating, and then the desired target product is prepared by heat treatment.
The products prepared by this method have the advantages of uniform chemical composition, high purity, narrow and uniform particle size distribution,
low heat treatment temperature, and precise control of chemical stoichiometry.
However, the drawbacks of this method are also evident, including difficulty in controlling the morphology of the product, high cost, complex operation, and difficulty in industrialization.
At present, this method is limited to laboratory research.
Luo et al. used the sol gel method to obtain sol by mixing LiAc · 2H2O, Co (Ac) 2 · 4H2O, Ni (Ac) 2 · 4H2O, Mn (Ac) 2 · 4H2O as raw materials, citric acid as complexing agent,
according to the stoichiometric ratio (Li: Ni: Co: Mn=1.05:0.5:0.2:0.3) of raw materials,
heating the water bath to remove the water in the sol to obtain gel, drying the gel at 450 ℃ for 5h, sintering it at 900 ℃ for 12h,
and then conducting double-sided coating modification. At 55 ℃, the voltage range is 2.5-4.5V, and the initial discharge specific capacity at 1C is 116.6mAh/g.
The capacity retention rate after 50 cycles is 74.2%, showing excellent electrochemical cycling performance compared to the sample before modification treatment.
Lithium nickelate is a positive electrode material with high energy density, low cost, and non toxicity.
The production process mainly includes synthesis, impurity removal, batching, sintering, and processing.
The synthesis methods of lithium nickel oxide mainly include solid phase method, liquid phase method and sol gel method.
The impurity removal and batching operations are similar to lithium cobalt oxide, with a higher sintering temperature, generally above 700 ℃.
After sintering, water washing treatment is required to remove impurities on the surface of the product.
The processing steps are similar to lithium cobalt oxide, including crushing, screening, mixing, pressing and forming operations.
Ternary materials are positive electrode materials with high energy density, long lifespan, and environmental friendliness.
The production process mainly includes synthesis, impurity removal, batching, sintering, and processing.
The synthesis methods of ternary materials mainly include solid phase method, liquid phase method and sol gel method.
The impurity removal and batching operations are similar to lithium cobalt oxide, with a higher sintering temperature, generally above 700 ℃.
During the sintering process, the atmosphere and pressure need to be controlled to ensure product performance and quality.
The processing steps are similar to lithium cobalt oxide, including crushing, screening, mixing, pressing and forming operations.
The key production equipment mainly includes reaction furnaces, high-temperature furnaces, crushers, mixers, tablet presses, etc.
The reactor is mainly used for the synthesis process, the high-temperature furnace is mainly used for the sintering process,
the crusher and mixer are mainly used for the batching and mixing process,
and the tablet press is mainly used for the processing process. The performance and quality of these devices directly affect the performance and quality of the product.






