New strategy enables lithium-ion batteries to achieve super fast charging
Aug,02,24
The team led by Professors Ji Xingxing and Wu Xiaojun from the University of Science and Technology of China,
together with Professor Duan Xiangfeng's team from the University of California,
Los Angeles, has broken through the traditional electrocatalytic model at the interface of solid-liquid and solid gas phases, and achieved a new "solid-state electrocatalysis".
This strategy has been successfully applied to the negative electrode material of pure solid-state reactions,
enabling lithium-ion batteries to achieve a high energy density of 302 watt hours per kilogram and charge to 80% in 9 minutes.
The relevant research results were recently published in the Journal of the American Chemical Society.
Negative electrode materials that store lithium ions through alloying reactions, such as silicon and phosphorus,
have significantly higher specific capacity compared to traditional graphite negative electrodes.
However, the slow lithiation reaction kinetics of these negative electrode materials during the storage of lithium ions limit the fast charging performance of the material system.
In electrochemical research, researchers often choose to use electrocatalytic strategies to improve reaction kinetics.
However, in the lithiation reaction process of alloying reaction electrode materials, the reactants and products are in complete solid-phase contact,
and there is no two-phase interface required for conventional electrocatalysis between the two.
Therefore, the reaction kinetics between negative electrode materials and lithium ions in catalytic alloying reactions are still a research gap.
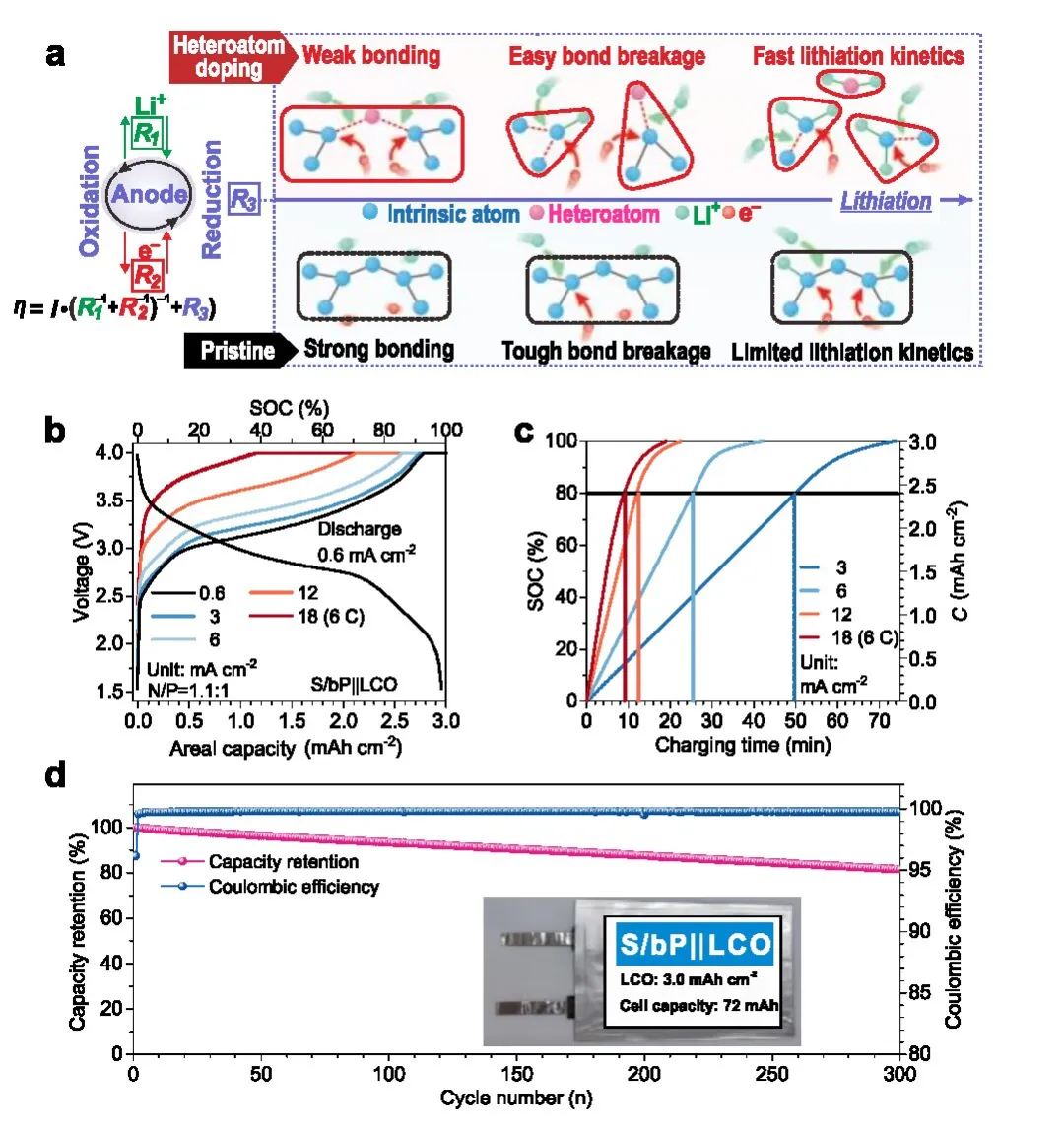
Figure 1: Design concept of solid-phase electrocatalysis and related soft pack battery performance
In response to the above issues, the research team proposed the conversion reaction rate of heterogeneous atom doping catalysis and evolutionary negative electrode materials.
By combining theoretical calculations and in-situ X-ray absorption spectroscopy testing, the research team obtained the following physical images:
a small amount of impurity doping can provide high reactive active sites for alloying reactions of alloy type negative electrode materials, promote intrinsic chemical bond breaking,
and continuously break bonds at the doping sites, splitting the negative electrode material into more and smaller structural units, providing more reaction sites for further reactions,
thereby reducing reaction impedance and improving reaction kinetics.
The soft pack battery assembled by combining a synthesized sulfur doped phosphorus negative electrode
and a commercial lithium cobalt oxide positive electrode has successfully achieved an energy density of 302 watt hours per kilogram, charging to 80% capacity in 9 minutes,
and the fast charging performance of the battery can stably cycle for more than 300 cycles.






