Structure and principle of lithium-ion batteries
Aug,02,24
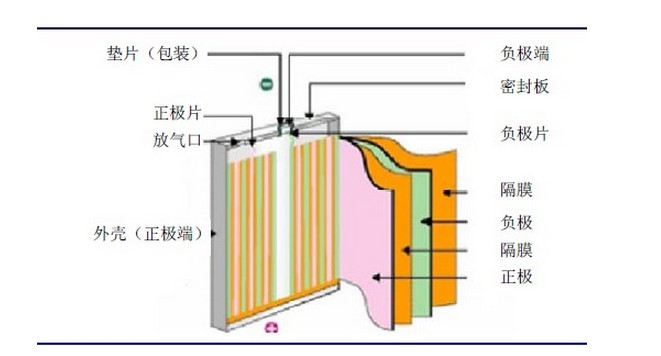
The main components of lithium-ion batteries are:
(1) Positive electrode - the active material mainly refers to lithium cobalt oxide,
lithium manganese oxide, lithium iron phosphate, lithium nickel oxide, lithium nickel cobalt manganese oxide, etc.
The conductive current collector generally uses aluminum foil;
(2) Diaphragm - a special plastic film that allows lithium ions to pass through, but is an insulator for electrons.
Currently, there are mainly two types and their combinations: PE and PP.
There is also a type of inorganic solid membrane, such as alumina membrane coating, which is a type of inorganic solid membrane;
(3) Negative electrode - the active material mainly refers to graphite, lithium titanate, or carbon materials with a graphite like structure,
and copper foil is generally used as the conductive current collector;
(4) Electrolyte - generally organic system, such as carbonate solvent dissolved with lithium hexafluorophosphate, and some polymer batteries use gel electrolyte;
(5) Battery shell - mainly divided into two types: hard shell (steel shell, aluminum shell, nickel plated iron shell, etc.) and soft shell (aluminum-plastic film).
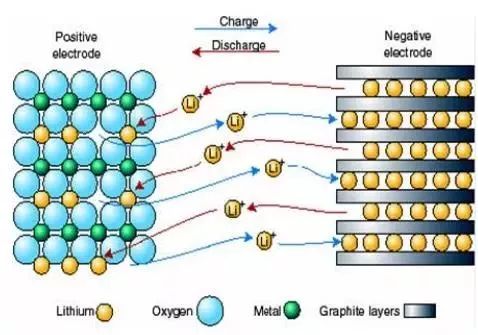
When the battery is charged, lithium ions are deintercalated from the positive electrode and intercalated in the negative electrode, and vice versa during discharge.
This requires an electrode to be in a lithium intercalated state before assembly.
Generally, a lithium intercalated transition metal oxide with a potential greater than 3V relative to lithium and stable in air is selected as the positive electrode, such as LiCoO2, LiNiO2, LiMn2O4.
As the material for the negative electrode, it is recommended to choose lithium compounds that can be embedded with a potential as close as possible to the lithium potential,
such as various carbon materials including natural graphite, synthetic graphite, carbon fibers, intermediate phase spherical carbon, and metal oxides,
including SnO, SnO2, tin composite oxide SnBxPyOz (x=0.4-0.6, y=0.6-0.4, z=(2+3x+5y)/2), etc.
The electrolyte adopts a mixed solvent system consisting of alkyl carbonates such as ethylene carbonate (EC), propylene carbonate (PC), and low viscosity diethyl carbonate (DEC) of LiPF6.
The membrane adopts polyolefin microporous membranes such as PE, PP or their composite membranes, especially the PP/PE/PP three-layer membrane,
which not only has a low melting point but also has high puncture resistance, playing a role in thermal insurance.
The shell is made of steel or aluminum material, and the cover component has the function of explosion-proof and power-off.
Basic working principle
When charging the battery, the lithium containing compound in the positive electrode releases lithium ions, which move through the electrolyte to the negative electrode.
The carbon material of the negative electrode has a layered structure with many micropores.
The lithium ions that reach the negative electrode are embedded into the micropores of the carbon layer.
The more lithium ions embedded, the higher the charging capacity.
When discharging the battery (i.e. the process of using the battery), the lithium ions embedded in the negative carbon layer are released and move back to the positive electrode.
The more lithium ions return to the positive electrode, the higher the discharge capacity. The battery capacity we usually refer to is the discharge capacity.
During the charging and discharging process of lithium-ion batteries, lithium ions move from the positive electrode to the negative electrode and then to the positive electrode.
This is like a rocking chair, with the two ends of the rocking chair being the two poles of the battery, and lithium ions moving back and forth between the two ends of the rocking chair.
So lithium-ion batteries are also known as rocking chair batteries.
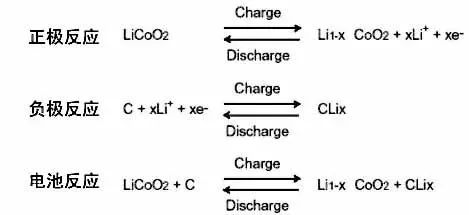
Charge discharge mechanism
The charging process of lithium-ion batteries is divided into two stages: constant current charging stage and constant voltage current decreasing charging stage.
Excessive charging and discharging of lithium-ion batteries can cause permanent damage to the positive and negative electrodes.
Excessive discharge leads to the collapse of the negative carbon layer structure, which can result in the inability of lithium ions to insert during the charging process;
Overcharging causes excessive lithium ions to embed into the negative carbon structure, resulting in some of the lithium ions no longer being able to be released.
The best charging and discharging method for lithium-ion batteries to maintain performance is shallow charging and shallow discharging.
Generally, 60% DOD is 2-4 times the cycle life under 100% DOD conditions.






