TEM analysis method for lithium battery materials
Aug,05,24
It is very important for Jinjian Laboratory to conduct research on the atomic and electronic structure of lithium battery materials.
Transmission electron microscopy plays a crucial role in this regard as it has atomic scale spatial resolution, which can help researchers observe and analyze the microstructure of materials.
Through transmission electron microscopy, researchers can observe subtle changes such as atomic arrangement, lattice defects,
and structural distortions in lithium-ion battery materials, thus better understanding the performance of the materials.
In addition, transmission electron microscopy can provide information about the electronic structure of materials,
helping researchers gain a deeper understanding of the electronic properties of materials, such as charge distribution and band structure.
By conducting in-depth research on the atomic and electronic structures of lithium-ion battery materials,
scientists can optimize material design, improve battery performance, enhance battery cycle life, energy density, and safety,
and promote the development and application of lithium-ion battery technology.
This micro level research is of great significance for improving battery performance and promoting the development of new energy fields.
TEM analysis method for lithium battery materials
1. TEM mode characterization
TEM modes are mainly divided into two categories: image modes and diffraction modes.
Patterns are commonly used to observe the morphology of samples. In addition,
high-resolution transmission electron microscopy (HRTEM) can be used to obtain atomic scale resolution structural images.
The diffraction mode usually uses selected area electron diffraction (SEAD) method to obtain the electron diffraction results of the selected area,
which can analyze the crystallinity and phase structure information of the selected location.
(a) TEM bright field photograph
(b) Selected electron diffraction images and high-resolution photographs
Figure 1 TEM characterization effect of ternary materials
2. STEM mode representation
The STEM mode scans the sample surface with a converging electron beam and uses a circular detector to receive scattered electrons at different receiving angles for imaging.
High angle annular dark field imaging (HAADF) and annular bright field imaging (ABF) are widely used in lithium-ion batteries. HAADF is sensitive to heavy elements.
ABF is sensitive to light elements and can be used for direct imaging of light elements such as Li and O. It is crucial for the research of lithium-ion battery materials, as shown in Figure 2.
(a) ABF image of original LiFePO4
(b) ABF image of LiFePO4 in completely delithiated state
(c) ABF image of LiFePO4 in semi delithiated state reveals the order structure of Li and Li vacancies
Figure 2 Atomic scale structure of LiFePO4 cathode material at different lithium removal rates
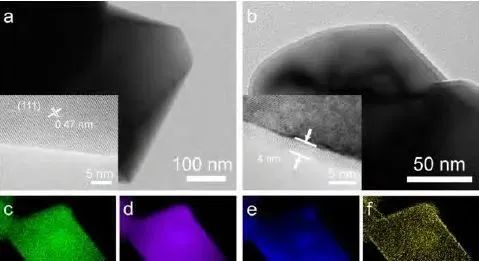
3. X-ray energy spectrum
When the excited electrons in the sample return to the ground state, they emit X-rays, which can be received to obtain an X-ray energy spectrum (EDS).
EDS analyzes the characteristic X-rays emitted from the surface of the sample to obtain information about the elements contained in the sample.
Collect energy spectrum information in TEM mode as the average result, reflecting the average elemental composition and proportion of the electron beam irradiation area.
In STEM mode, the relationship between element types and element positions can be established, and the distribution map of elements can be obtained, as shown in Figure 3.
4. Electronic holography
Electronic holography can study the potential distribution of materials, which is of great significance for lithium battery materials.
The holography referred to here usually refers to off-axis holography,
which means that half of the incident electron beam passes through the sample and half passes through the vacuum,
thereby forming object waves and reference waves. As shown in Figure 4,
the object wave and the reference wave interfere with each other after being deflected by an electron prism to form a holographic pattern.
Perform data processing in the later stage to reconstruct the pattern and obtain the potential distribution.
he potential distribution changes of battery materials during cycling can be obtained through electronic holography.
(a) The object wave and reference wave interfere with each other through the action of an electron prism to form a holographic pattern
(b) Reconstruct object waves by performing Fourier transform on holographic patterns. The phase of the reconstructed object wave is the potential distribution
Figure 4 Schematic diagram of electronic holography
5. Electronic energy loss spectrum
After the electron beam passes through the sample in a transmission mirror, scattering occurs.
The energy of the electrons undergoing elastic scattering remains constant, while the energy of the electrons undergoing inelastic scattering changes.
The Electronic Energy Loss Spectroscopy (EELS) analyzes the energy loss distribution of incident electrons with fixed energy after inelastic scattering from the sample.
Non elastic scattering involves Coulomb interactions between electrons and electrons outside the sample nucleus,
where electrons outside the nucleus undergo selective transitions upon receiving energy from incident electrons,
while the incident electrons lose the corresponding energy. The energy required for selective transitions of different elements in different states varies.
Therefore, based on the energy lost by incident electrons, the elemental information and electronic structure information of the sample can be obtained,
including obtaining sample thickness, distinguishing element types and contents, determining element valence states, and other structural information.
The differences between EELS and EDS are shown in Table 1:
Figure 5 Analysis of Carbon Bond Environment near Branches
6. Convergent beam electron diffraction
Convergent beam electron diffraction (CBED) can obtain structural information of electron orbital levels.
CBED measures the Fourier coefficient of the Coulomb potential (structure factor) of a crystal,
which is then converted into the X-ray structure factor, and the electron density is obtained through Fourier transform.
Electron diffraction measurement of structural factors has the advantages of being able to measure low order structural factors,
being sensitive to electronic states, and enabling precise analysis of micro areas, ensuring the accuracy of obtaining electron density.
The electron density can be obtained through multipole fitting to obtain information such as crystal orbitals and topological states.
The charge density and bonding behavior in LiNiO2 material are shown in Figure 6.
Due to the fact that the CBED method requires the electron beam to act on the sample for a long time and at a high dose,
it cannot be widely applied in the research of lithium battery materials at present.
Figure 6 (a) CBED pattern of LiNiO2 material
(b) The best fit between refined experimental data and theoretical calculation results
7. Cryo electron microscope
Lithium ion battery materials are usually very sensitive to electron beam irradiation, such as metal lithium anodes and solid electrolyte materials,
which limits the study of many electron beam sensitive materials by electron microscopy.
Recently, Professor Cui Yi's team from Stanford University and Professor Meng Ying's team from the University of California,
San Diego respectively used frozen sample rods to characterize metallic lithium using HRTEM at liquid nitrogen temperature.
Electrolyte is an important component of lithium-ion batteries, but due to the fact that most electrolytes are liquid,
research on the structure and performance of liquid electrolytes is very insufficient.
Recently, thanks to the development of cryo electron microscopy and cryo FIB,
it has become possible to study the state of liquid electrolyte systems during different charge and discharge processes in electron microscopy, as shown in Figure 7.
Figure 7 (a) FIB image of Type I crystal, SEI film, and electrolyte
(b) FIB images of type II crystal and electrolyte
(c) HAADF Cryo STEM images of Type I crystal, SEI film, and electrolyte
(d) HAADF Cryo STEM Images of Type II Crystallization and Electrolyte
8. In-situ power up
The life of a lithium battery is spent in cycles of charging and discharging, so in-situ characterization during the charging and discharging process is crucial.
In 2009, Allard et al. used microelectromechanical systems (MEMS) chips to carry samples and designed a new in-situ sample rod that achieved rapid heating and cooling processes,
with a maximum temperature exceeding 1000 degrees Celsius.
At the same time, the stability of the sample rod was sufficient to ensure the acquisition of atomic scale images under STEM;
Gong Yue et al. applied the chip type sample rod to the in-situ study of lithium-ion battery materials,
successfully building a micro all solid state battery on the in-situ chip and achieving in-situ observation of lithium-ion migration at the atomic scale (see Figure 8),
further expanding the characterization range to the three-dimensional atomic scale.
Chip based sample rods have the advantages of tilting, high stability, strong operability, and ease of further processing, and have become the mainstream in in-situ research.
(a) SEM image of all solid state lithium-ion battery constructed using FIB
(b) Schematic diagram of the constructed all solid state battery
(c) Atomic scale ABF image of raw LiCoO2 cathode material
(d) Atomic scale HAADF image of the original LiCoO2 cathode material
Figure 8: Initial Structure of Microscopic All Solid State Battery Materials
9. In-situ temperature variation
Temperature can have an impact on the performance of batteries in practical applications,
and the performance of batteries at high or low temperatures is crucial for their practical application promotion.
The heating and low-temperature tests in in-situ electron microscopy use different principles.
Heating is controlled by the heat generated by electric current,
while low temperature is achieved by balancing liquid nitrogen and electric heating to bring the sample between room temperature and liquid nitrogen temperature.
Figure 9 shows the structure of electrode materials at different temperatures, which is of great significance for understanding the performance of lithium batteries in practical working environments.
In the future, we hope to combine in-situ temperature change and charging, which will have more practical value and significance for the research of lithium battery materials.
(a) HRTEM image before heating
(b) HRTEM image after heating at 100 ℃
(c) HRTEM image after heating at 200 ℃
(d) HRTEM image after heating at 300 ℃
The inserted small images in each picture represent the selected electron diffraction patterns of the samples at their respective temperatures
Figure 9 HRTEM image of overcharged Li0.15Ni0.8Co0.15Al0.05O2 particles
10. 3D reconstruction
There are usually two methods to obtain three-dimensional structural information in electron microscopy.
One is to record the structural information of the sample at different angles by tilting the sample in an electron microscope,
and then restore the three-dimensional structure of the sample;
Another method is to reconstruct the three-dimensional structure of the sample through the method of outgoing wave reconstruction.
The schematic diagrams of the two methods are shown in Figure 10.
At present, the atomic scale 3D reconstruction method has strict requirements for samples and has not yet been applied in lithium battery materials.
However, through multi-directional structural characterization of the sample, three-dimensional structural information hidden behind the two-dimensional projection results has been discovered.
I believe that with the advancement of technology, 3D reconstruction methods can achieve fruitful results in the research of lithium battery materials in the future.
Figure 10 (a) Three dimensional reconstruction method for continuously tilted samples
(b) Method for reconstructing three-dimensional structures using outgoing waves






