Introduction to Lithium Manganese Iron Phosphate LMFP Material
Aug,05,24
1、 Lithium manganese iron phosphate LMFP has cost-effectiveness advantages
The energy E of a lithium battery is equal to the product of the average operating voltage 𝑉𝑎𝑣𝑒 and the mass (volume) specific capacity 𝑄𝑚𝑎𝑥, i.e. 𝐸=𝑉𝑎𝑣𝑒∗𝑄𝑚𝑎𝑥.
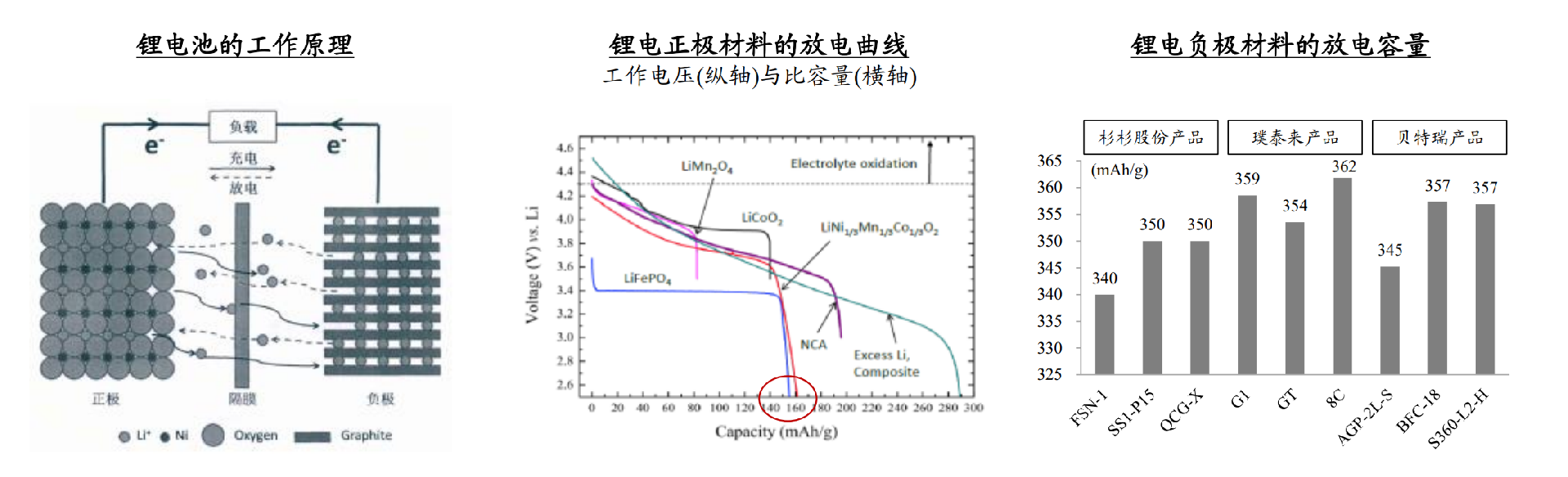
Figure 1 Working principle of lithium battery, discharge capacity of positive and negative electrode materials (unit: mAh/g, V)
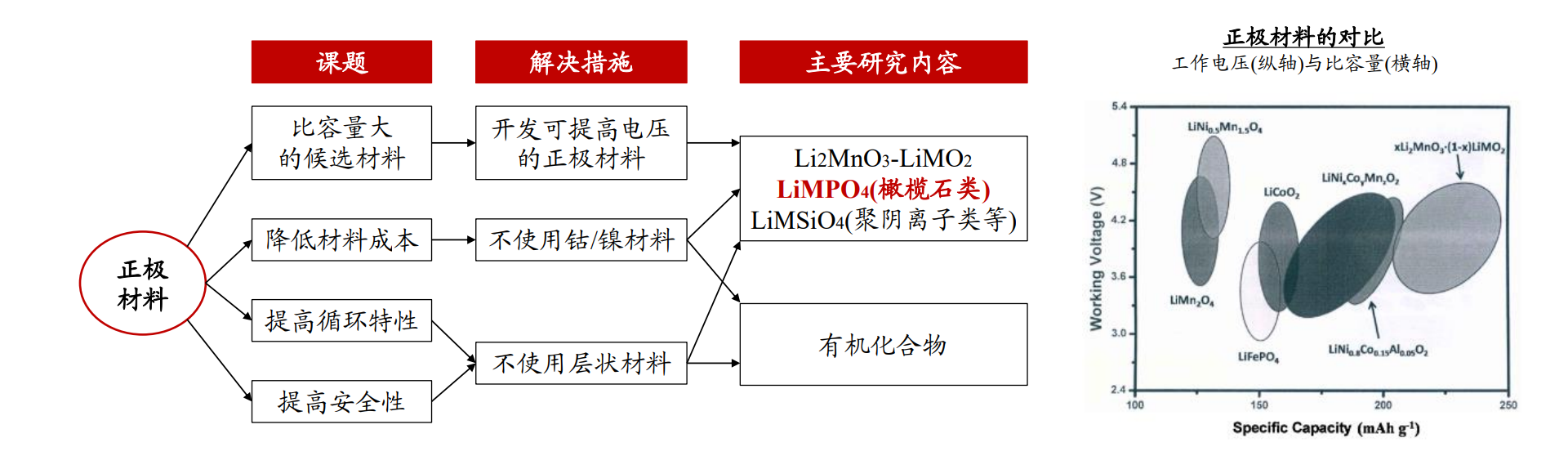
The ideal positive electrode material should have the following characteristics:
The discharge platform is high and stable, and does not react with the electrolyte.
The crystal structure is stable, and the change in redox potential during charging and discharging is small.
A higher lithium ion diffusion coefficient can reduce polarization, minimize energy loss, and achieve faster charging and discharging.
There is a large Gibbs free energy in lithium-ion reactions to reduce energy loss caused by polarization.
Figure 2 Comparison of research directions, voltage, and specific capacity of lithium-ion cathode materials (unit: mAh/g, V)
The LFP positive electrode has many advantages such as good electrical performance, low cost, non toxicity, good thermal stability, and environmental friendliness.
However, the low potential (with a flat discharge plateau at around 3.4V) results in lower energy density and lower intrinsic electronic conductivity.
Therefore, doping transition metals such as Co, Mn, and Ni to improve the voltage plateau of LFP and increase energy density has become one of the key research directions.
Figure 3: Performance comparison of different types of positive electrode materials (unit: cm ^ 2/s, S/cm,mAh/g,Wh/kg)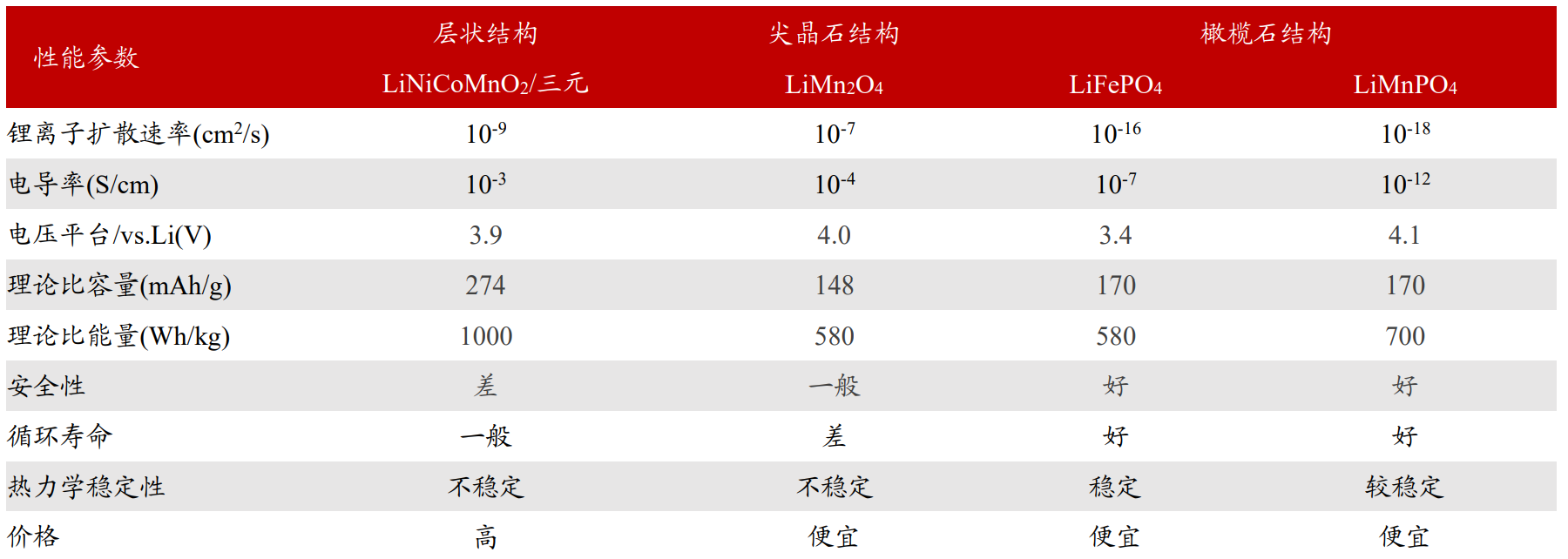
Doping with Mn is preferred, as it can combine the high conductivity of LFP with the high voltage of LMP.
Cobalt and nickel: Although the theoretical capacities of LiCoPO4 (LCP, 4.8V) and LiNiPO4 (LNP, 5.2V) are similar to LFP,
their operating voltages exceed the electrolyte voltage range, and their costs are also high.
Therefore, LCP and LNP have no industrial significance.
Vanadium is highly toxic, expensive, and lacks outstanding electrochemical performance, making it difficult to commercialize Li3V2 (PO4) 3 (LVP, 4.0V/3.7V/3.6V).
Manganese: The electrode potential of LiMnPO4 (LMP) relative to Li+/Li is 4.1V, while that of high LFP is 3.4V.
Therefore, the theoretical mass energy density of LMP material is about 21% higher than that of LFP;
The structure is basically the same, only the lattice parameters are different (Fe2+radius is 0.092nm, Mn2+radius is 0.097nm).
Therefore, doping manganese in LFP forms a multi-component (LiMnxFe1-xPO4/LMFP or LFMP) system,
which has better low-temperature performance. But there are problems such as low conductivity, poor rate performance, and poor cycling performance.
Figure 4 Comparison of Crystal Structure and Properties between Lithium Iron Phosphate and Lithium Manganese Iron Phosphate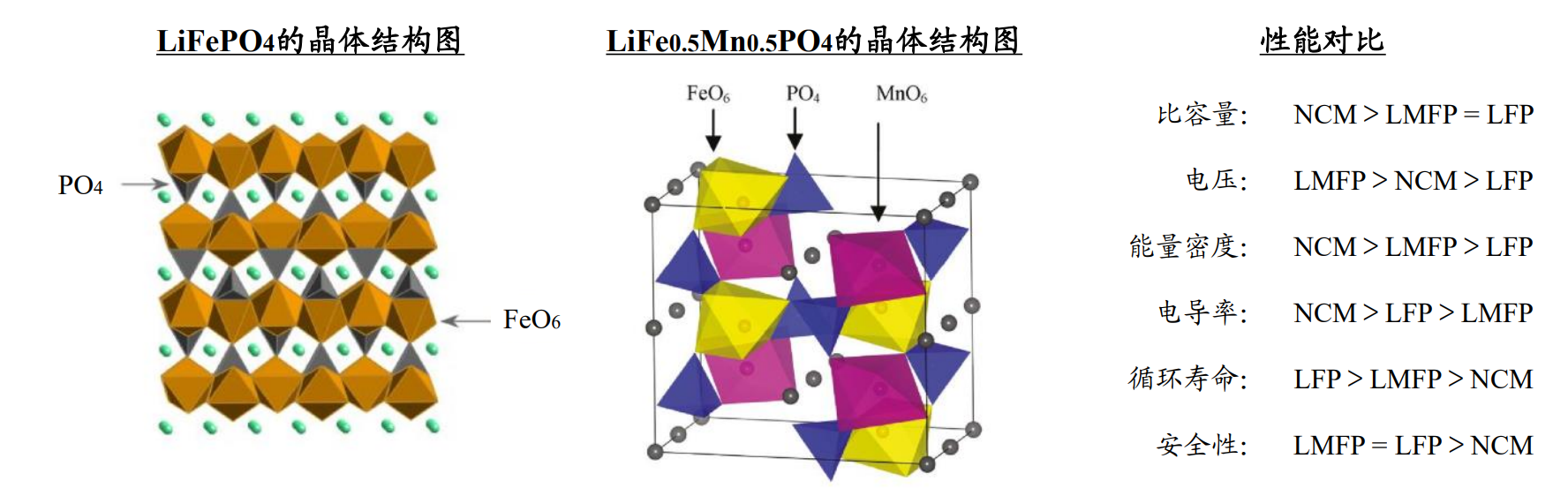
2、The proportion of Mn doping affects the performance
The doping amount of Mn is too high. Due to the John Teller effect of Mn element,
the discharge specific capacity of LMFP material is low and decays rapidly, resulting in a low capacity retention rate
If the doping amount of Mn is too low, the LMFP material cannot significantly increase the platform voltage, thus unable to obtain the maximum discharge specific energy.
Figure 5 Comparison of LMFP indicators with different ratios of 0.1C current density (unit: mAg/g, V, times)
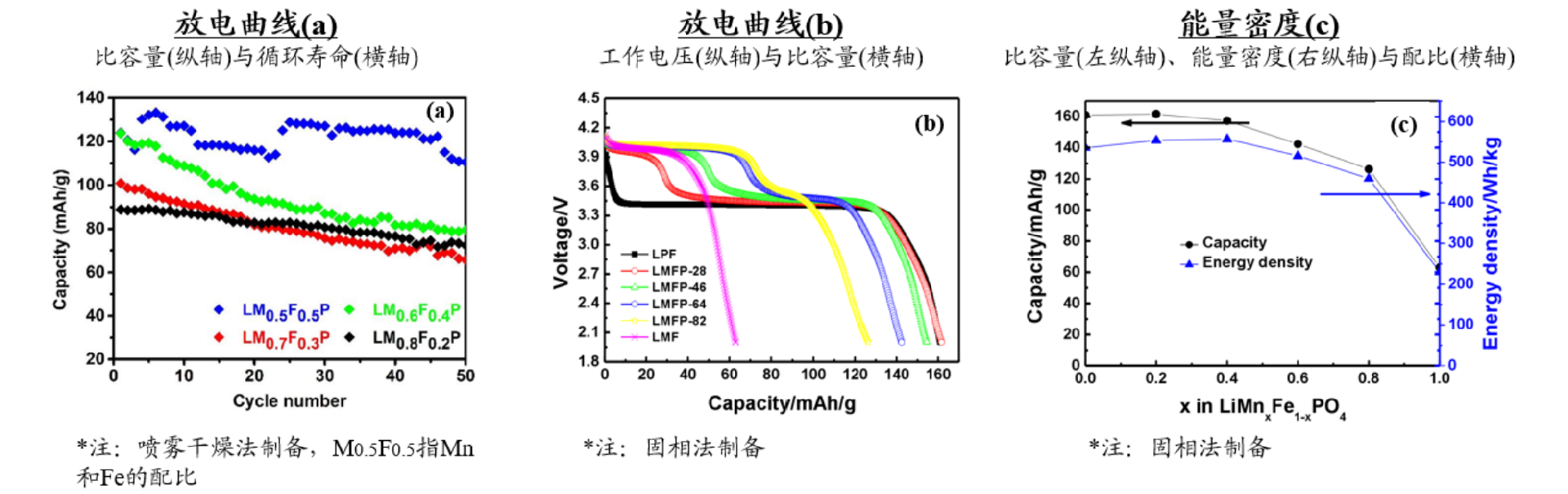
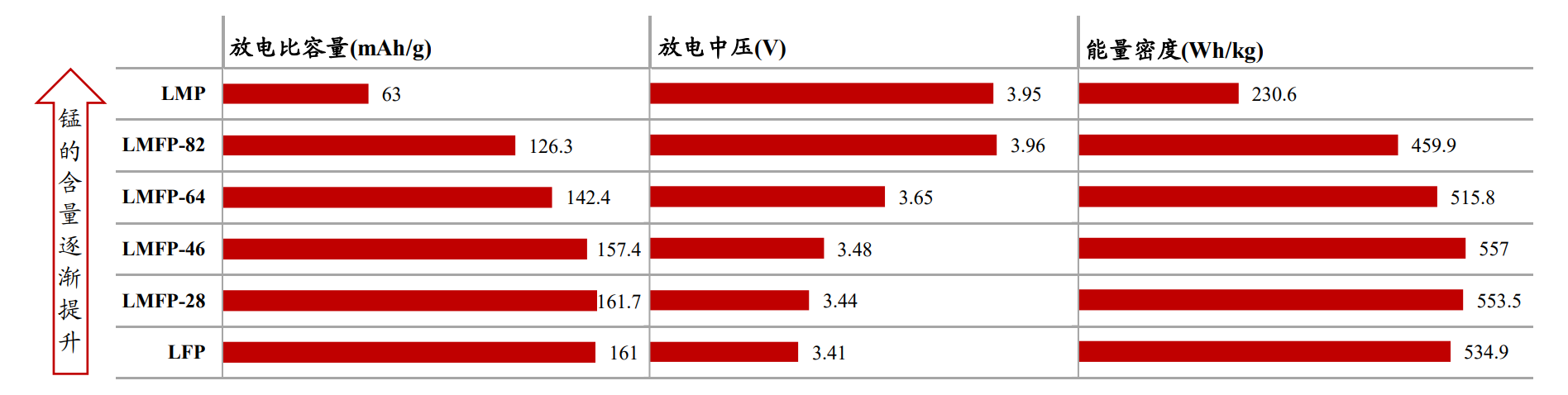
Figure 6 Comparison of LMFP, LMP, and LFP indicators with different ratios at a current density of 0.1C (unit: mAg/g, V, Wh/kg)
3、Technological modification to provide diversified applications
The LMFP material mainly has the following problems:
Lower conductivity and ion diffusion coefficient than LFP, the latter affecting charge discharge rate characteristics
The Dual Voltage Problem of Mn and Fe
Poor cycling performance
The problem of manganese dissolution in electrolyte, etc.
These issues will affect the performance of LMFP materials, and existing technologies mainly focus on modification techniques such as carbon coating, nanomaterialization,
and lithium supplementation, as well as composite with ternary materials, in order to obtain better performance and industrial technology.
(1) The main principle of coating carbon layer modification is to:
(1) form a good conductive network through the mutual contact between the carbon layer and particles, thereby improving the electronic conductivity of the material;
(2) Prevent further growth of lithium manganese iron phosphate particles to improve battery performance.
(3) Prevent hydrogen fluoride in the electrolyte from corroding the positive electrode material, thereby improving the cycling performance of the positive electrode material.
Figure 7 Charge discharge curves and rate performance of LMFP with different carbon contents (unit: mAg/g, V, times)
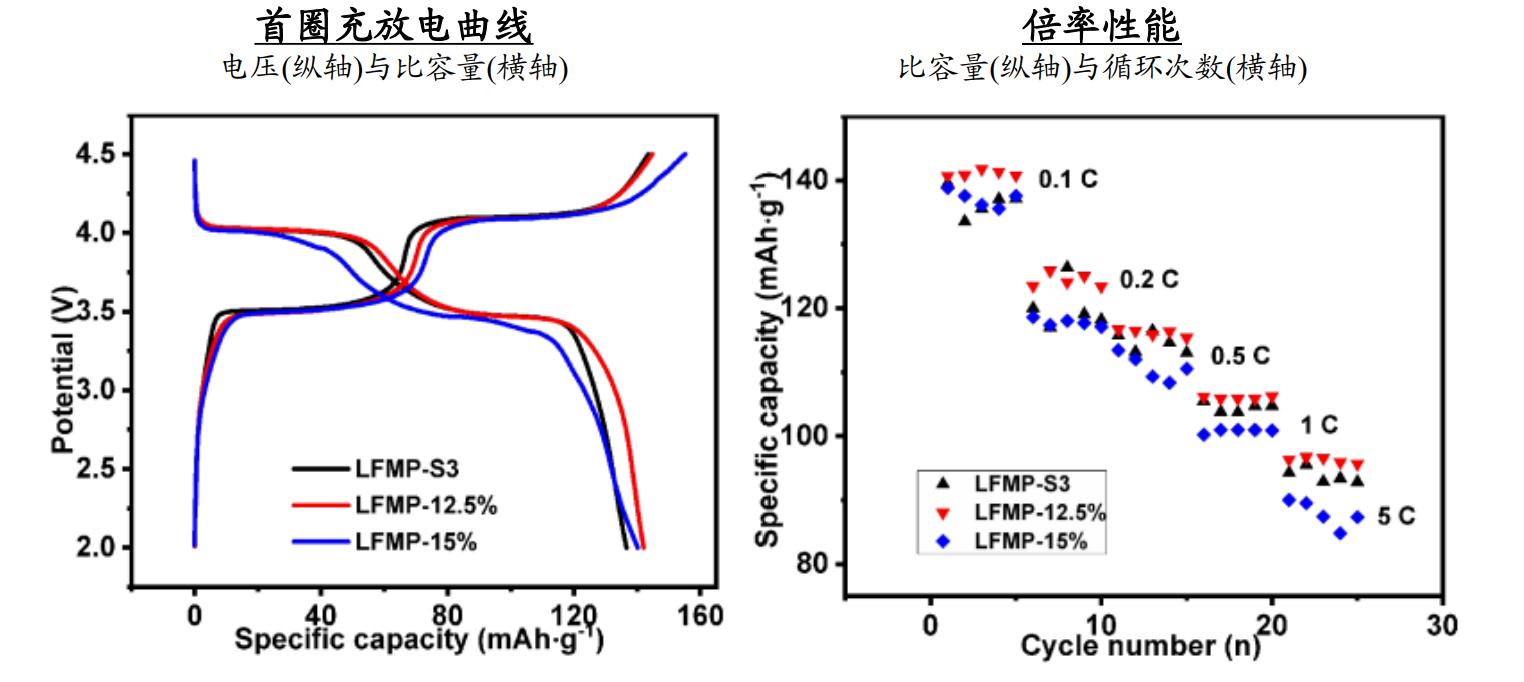
*Note: Prepared by high-temperature solid-phase method, the material in the figure is LiMn0.5Fe0.5PO4/C,
and the left figure shows the first cycle charge discharge curve at a rate of 0.1C.
Among them, LFMP-S3, LFMP-12.5%, and LFMP-15% refer to the carbon source (sucrose) raw material with mass contents of 10%, 12.5%, and 15%, respectively.
The actual carbon content is 3.68%, 4.8%, and 6.57%, respectively.
(2) Nanotechnology is an important means to improve conductivity and other properties.
The diffusion coefficient of lithium ions is directly determined by the particle size,
and the ion diffusion coefficient at the nanoscale is much larger than that of particles at the micrometer and larger sizes,
because reducing the particle size can shorten the diffusion path of ions and improve conductivity and other properties.
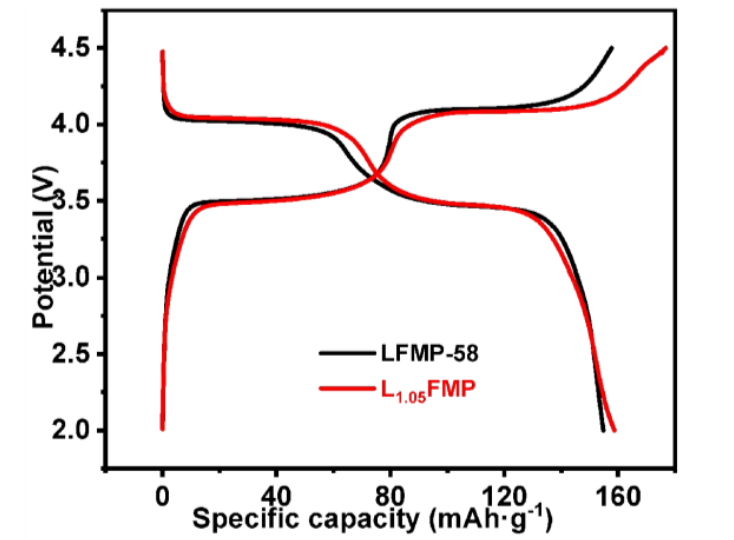
(3) LMFP+lithium supplements are used to improve material properties.
There are mainly two paths, one is that excessive lithium element will coat a layer of conductive material on the surface of LFP particles to enhance the electrochemical performance of the product;
Secondly, excessive lithium element can reduce the anti site defects of iron and lithium (which can block the diffusion channels of lithium ions),
thereby enhancing the electrochemical performance of the product.
Figure 8 Charge discharge curves of LMFP and LFMP with excess lithium (unit: mAg/g, V)