Failure analysis of lithium-ion batteries!
Aug,08,24
The failure analysis of lithium-ion batteries can generally be divided into four sections:
determination of failure analysis process, identification of failure phenomena, failure analysis testing, and failure mechanism analysis.
1. Failure analysis process of lithium-ion batteries
For batteries that have already experienced significant failure,
it is necessary to predict the cause of the failure based on the failure phenomenon that has occurred,
and determine the testing and characterization required from the outside in.
Due to the limited number of sample batteries (which have experienced a specific failure) that can be used for testing,
and the fact that the internal components that can provide information about the failure are also related to the battery itself,
the testing and characterization of the battery need to consider whether it will damage its original state after the failure and result in the loss of some information.
According to the impact of test characterization on the sample, testing methods can be divided into two categories: "lossy" and "non-destructive" (or in situ and non in situ).
Non destructive testing is the testing and characterization of a sample without compromising its overall integrity and failure state.
It mainly includes direct testing of the electrochemical performance of the battery and in-situ characterization (such as in-situ XRD) that can be performed without disassembling the battery.
Destructive testing requires disassembling the battery and conducting targeted testing on its components,
including direct testing and characterization of active materials such as electrode plates, separators, electrolytes, etc.
Due to the impact of some testing methods on the original failure state of the battery, it is necessary to plan the testing sequence,
which can be divided into four steps: visual inspection, non-destructive testing, destructive testing, and comprehensive result analysis, as shown in Figure 1.
The information obtained in each step can assist in predicting the cause of battery failure and guide the testing methods that should be used in the next step.
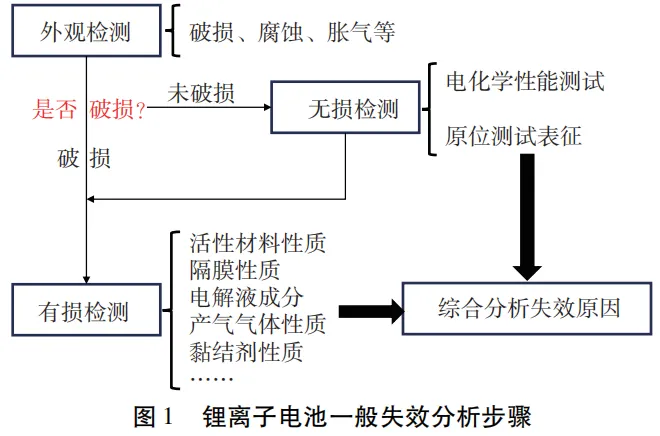
Table 1 provides the basic steps and the testing and characterization carried out, but specific samples still need to be selected and combined according to the actual situation. After completing all tests and analyzing the results from various aspects, the reasons for battery failure and the roles played by each component in the process can be determined.
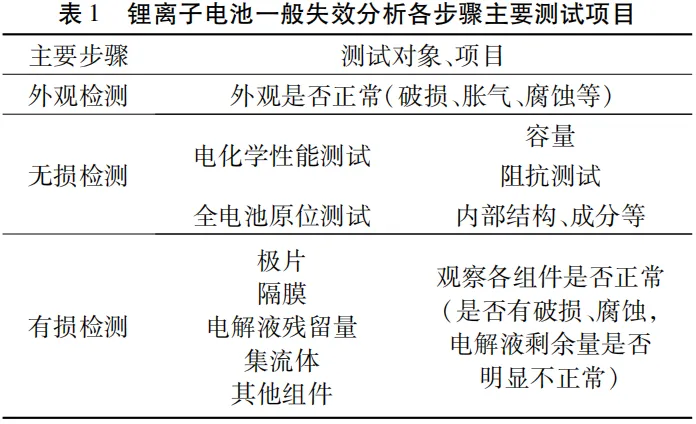
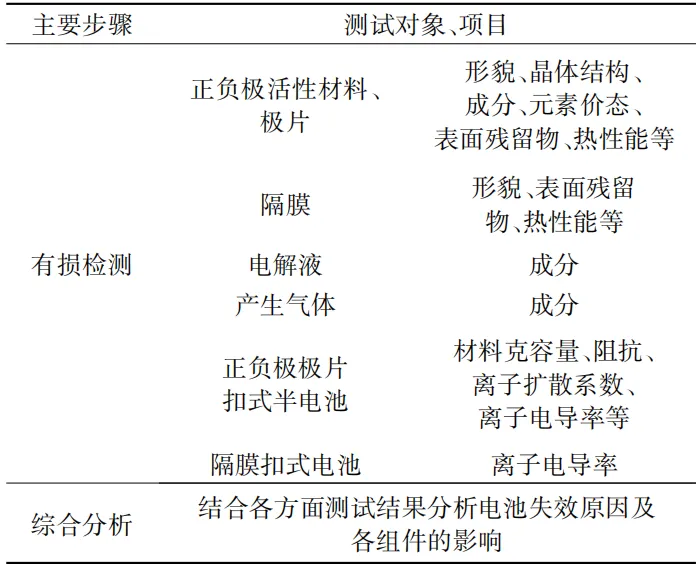
1.1 Appearance inspection
Inspect the appearance of the battery to determine if there are any damage, corrosion,
or burning issues that affect its integrity and prevent it from being charged or discharged.
It can also identify some external environmental conditions that the battery has experienced, such as needle puncture, compression, overheating, etc.
If it does not exist, non-destructive testing can continue;
If it exists, it is difficult to characterize the electrochemical performance and can only be partially characterized by in-situ testing or directly subjected to destructive detection.
1.2 Non destructive testing
To ensure that the battery is intact and there are no serious issues affecting its operation or safety, electrochemical performance tests can be conducted,
such as testing its remaining capacity, internal impedance, etc.
Through electrochemical performance tests, key data can be obtained to help determine the cause of its failure.
In addition, some in-situ testing methods can be used to obtain as much internal information as possible
without damaging the battery (such as whether there is damage inside and the amount of active material inside),
in order to help determine the possible causes of its failure and determine subsequent testing characterization methods.
By conducting visual inspection and non-destructive testing, it is possible to roughly predict the direction of battery failure.
Table 2 lists some possible failure phenomena corresponding to the results of appearance and non-destructive testing.
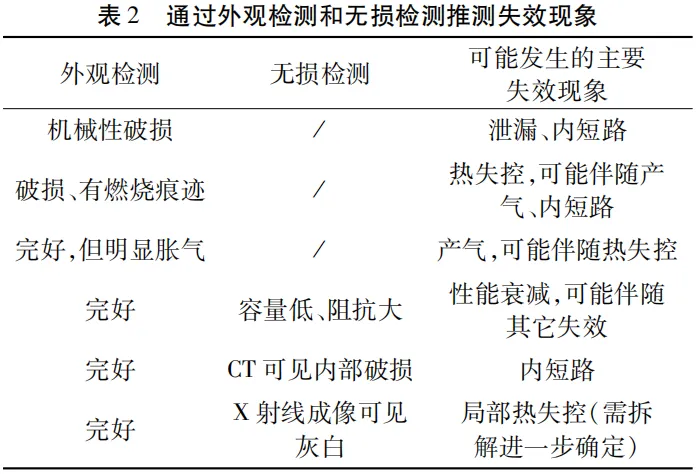
It should be mentioned that thermal runaway is a very serious and rapidly developing failure phenomenon.
In battery modules composed of a large number of lithium-ion batteries,
only one or a few lithium-ion batteries initially experience thermal runaway, but due to the domino effect,
it rapidly develops into thermal runaway in all lithium-ion batteries in the module.
Therefore, real-time detection is needed for this situation of thermal runaway.
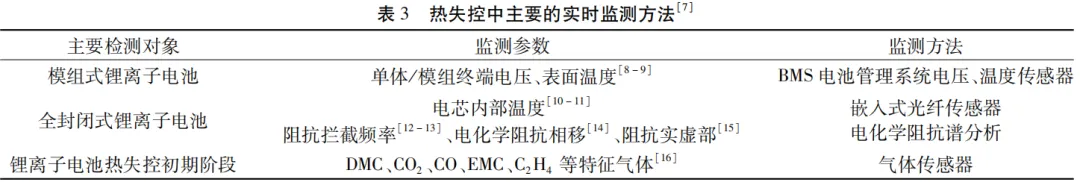
Based on the fact that thermal runaway is often accompanied by short circuits, high temperatures, and gas production, it is generally necessary to perform:
① real-time detection of individual/module terminal voltage and surface temperature;
② Real time monitoring of internal temperature and strain in lithium-ion batteries;
③ Monitoring of characteristic gas production components that may occur in lithium-ion batteries. The main methods are shown in Table 3.
1.3 Damage detection
After determining whether the appearance is damaged or completing non-destructive testing,
the battery is disassembled to obtain its internal components, and then targeted testing and characterization are carried out.
One thing to note is the pre-treatment method for the components, for example:
① Inert atmosphere protection is required when disassembling the battery;
② Separation and cleaning of active materials, current collectors, and binders;
③ When removing residual electrolyte on the diaphragm, prevent possible by-products from being removed;
④ Collection of electrolyte/gas during disassembly, etc.
The disassembled test objects mainly include positive and negative electrode plates, separators, electrolytes, generated gases, current collectors, and other components.
Among them, the properties of the active material (morphology, structure, composition, etc.) need to be characterized for the positive and negative electrode plates,
and then assembled into a button half cell for performance testing (gram capacity, impedance, etc.).
In addition, specific analysis can be conducted by separating adhesives, active materials, etc. based on the manifestation of failure.
The separator needs to undergo morphology and surface element analysis,
and can also be assembled into a button cell for performance testing (by analyzing its ion conductivity through impedance, etc.).
For electrolytes, gases, and possible residues on the surface of electrodes and separators, component analysis is required,
combined with analysis of possible reactions that may occur inside the battery.
1.4 Comprehensive analysis
After completing the damage detection, combined with testing the overall performance of the battery (if any) and testing and characterizing each component,
comprehensively analyze the types of failures that occur in the battery and the specific causes.
Main failure phenomena and causes of lithium-ion batteries
According to the nature of the main impact of lithium-ion battery failure, it can be divided into two aspects: battery performance and usage safety.
In terms of battery performance, it mainly includes two manifestations: capacity degradation and impedance increase,
which are also the most common failure modes in actual battery applications;
In terms of safety, there are manifestations such as bloating, thermal runaway, and internal short circuits.
2.1 Capacity attenuation
In the use of lithium-ion batteries, capacity decay is the most obvious characteristic of aging and failure, which can be divided into reversible decay and irreversible decay.
Among them, reversible capacity decay is mainly caused by the usage conditions of the battery
(such as the battery having extremely low capacity or even unable to work at low temperatures, and can continue to work normally when it returns to normal room temperature),
which can be restored to normal state after adjusting the battery usage and improving the environment;
Irreversible capacity decay is caused by irreversible changes in its internal components, resulting in an irreversible decline in performance.
For reversible decay, the battery charging and discharging voltage range, operating temperature, etc.
can be adjusted according to specific usage conditions to make it in the optimal state,
and after continuous use, it can be left to stand for a period of time to alleviate capacity decay caused by polarization and other factors.
For irreversible decay, the main reasons are the loss of lithium ion inventory and the failure of positive and negative electrode active materials.
Generally speaking, the charging and discharging process of lithium-ion batteries is related to the insertion/extraction of lithium ions on the positive and negative active materials,
so the capacity of the battery directly depends on the amount of active materials and available lithium ions.
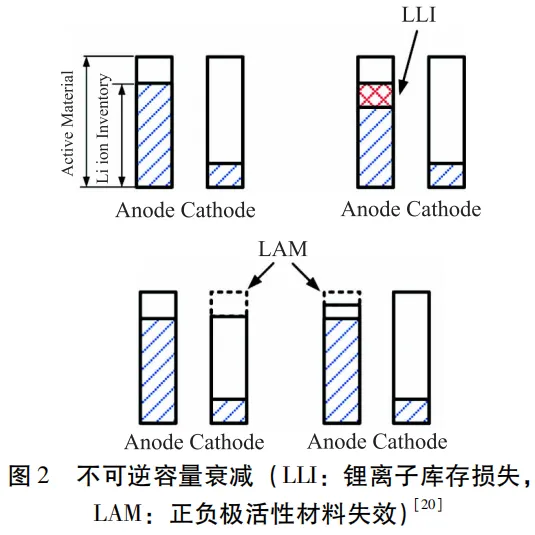
The process of charging and discharging a battery can be described as the process of two water cups pouring water into each other (as shown in Figure 2),
where the amount of active material is the volume of the water cup, and the amount of available lithium ions is the amount of water.
So the failure of the active material will lead to a decrease in the volume of the water cup,
and the reduction of available lithium ions will lead to a decrease in the amount of available water.
Both reasons will result in a decrease in the amount of water that can move between the two water cups.
For the failure of active materials, there are particle breakage, irreversible phase transition,
and mixed discharge of metal elements and lithium in the positive electrode material;
The negative electrode material mainly includes excessive growth of surface SEI film and huge volume expansion.
The loss of lithium-ion inventory is mainly caused by the reaction and decomposition of the electrolyte,
resulting in the conversion of available active lithium ions into by-products such as LiF and Li2CO3.
In addition, corrosion of current collectors such as aluminum foil and copper foil can cause the detachment of active substances, resulting in irreversible capacity loss.
2.2 Impedance increase
As a parameter directly related to the electronic and ion transport inside lithium-ion batteries,
the internal resistance of lithium-ion batteries is related to many factors such as their state of charge (SOC), number of cycles, and usage environment.
Moreover, an increase in internal resistance can also lead to a decrease in battery capacity.
Under the condition of constant charge and discharge cut-off voltage, the available capacity of the battery will decrease.
From the perspective of internal components of batteries,
the main reasons for impedance increase are: damage to positive and negative electrode active materials, excessive growth of SEI/CEI membranes,
electrolyte reactions and decomposition, adhesive failure, and separator failure.
Among them, the destruction of positive and negative electrode active materials naturally leads to the destruction of some formed lithium ion transmission paths,
resulting in a decrease in ion conductivity and an increase in impedance for lithium ion diffusion and migration;
The excessive growth of SEI and CEI films first leads to an increase in the path length of lithium ions during transport, resulting in an increase in impedance passing through.
In addition, the continued growth of SEI and CEI films will consume the active lithium ions in the electrolyte,
and the ion conductivity will decrease in the later stages of electrolyte reaction and decomposition aging,
resulting in increased resistance to the transfer of lithium ions in the electrolyte;
After the adhesive fails, it will cause the active substance to detach from the current collector
and the contact with the conductive additive to deteriorate, resulting in a decrease in electronic conductivity;
After the aging of the diaphragm, the pores through which ions pass may shrink or even directly block,
and by-products of the electrolyte may also adhere to them, resulting in increased resistance for lithium ions to pass through.
2.3 Safety performance (gas production, thermal runaway, internal short circuit)
Gas production is mainly caused by the presence of trace amounts of water in the battery system or factors such as high temperature, overcharging and discharging.
Trace amounts of water react with electrolytes during electrochemical processes to produce different gases, as shown in Figure 3.
Wu Kai et al. proposed the mechanism of electrolyte decomposition.
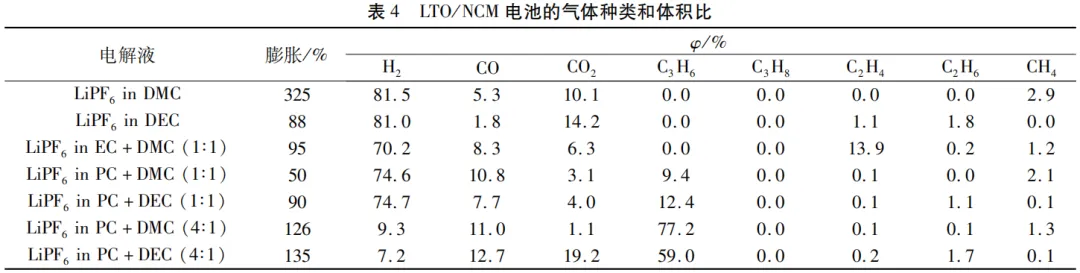
Table 4 shows the amount of different gases caused by different electrolytes in the battery system.
At high temperatures, different electrode materials and electrolytes with different compositions undergo electrochemical decomposition,
producing different gases including CH4, C2H4, C3H6, H2, CO2, etc.
Thermal runaway refers to the rapid accumulation of heat inside lithium-ion batteries without timely heat dissipation,
resulting in a rapid increase in temperature and further reactions.
The main reason for thermal runaway is that the battery operates under abnormal conditions, including short circuits, high temperatures, and external pressure.
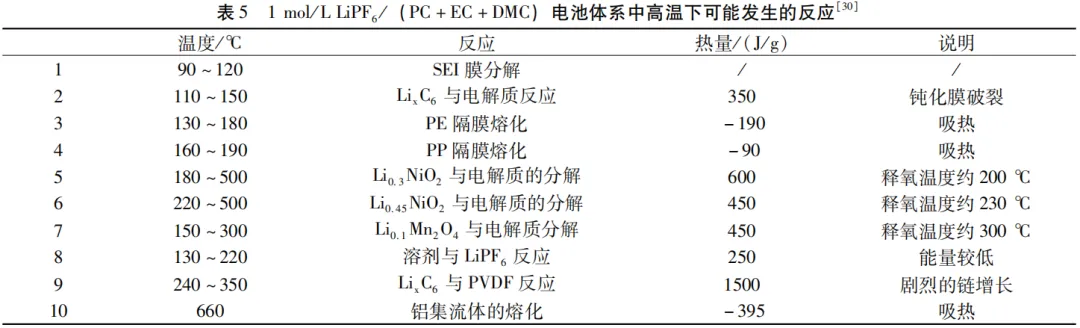
Due to the fact that most of the components inside the battery are in an unstable state at high temperatures (Table 5),
the battery is prone to combustion and even explosion in the event of thermal runaway.
Short circuit is a very dangerous situation in electricity.
For lithium-ion batteries, internal short circuits not only cause self discharge, but also lead to thermal runaway,
and more seriously, can develop into safety accidents such as fire and explosion.
The main causes of short circuits in lithium-ion batteries are short circuits between current collectors, membrane failure, and lithium dendrites.
The direct short circuit of the current collector mainly occurs during the production process,
where errors occur during battery packaging, resulting in displacement of the separator and failure to separate the positive and negative electrode plates, causing a short circuit.
Diaphragm failure is caused by aging of the diaphragm due to high temperature or electrolyte reaction,
resulting in the diaphragm losing its ability to separate positive and negative electrode plates.
During long-term cycling, the formation of lithium dendrites may puncture the membrane, leading to short circuits.
For negative electrode materials with lower discharge platforms like graphite, this situation is more pronounced.
In addition, overcharging and overdischarging during use may cause corrosion of the current collector and deposition on the electrode surface, leading to short circuits.
3 Failure testing methods for lithium-ion batteries
Regarding the analysis of the causes of battery failure,
based on the common manifestations of lithium-ion battery failure mentioned above, there may be some subtle phenomena.
Therefore, it is necessary to conduct electrochemical performance testing on the entire battery
(for example, if the battery only experiences impedance increase and there are no obvious phenomena in other aspects, impedance testing of the battery is required),
in order to understand the main phenomena of battery failure. From the perspective of the battery itself,
the main cause of battery failure is changes or even failures in its internal components.
Therefore, it is necessary to test the performance and properties of each component of the battery.
Ling Shigang et al. summarized some major and common testing methods for the electrochemical performance of lithium-ion batteries.
Generally, kinetic parameters related to the electrochemical process of lithium-ion batteries can be calculated and fitted using cyclic voltammetry (CV),
electrochemical impedance spectroscopy (EIS), constant current intermittent titration (GITT),
constant potential intermittent titration (PITT), potential relaxation technique (PRT), etc., including impedance parameters,
lithium-ion chemical diffusion coefficient, ion conductivity,
and electrode reaction rate constant. Li Wenjun et al. summarized some common methods for testing
and characterizing the different properties of lithium-ion batteries from the perspective of their internal components.
Mainly including the characterization of composition, morphology, structure, functional groups,
ion transport and other properties The composition and elemental states of active materials
and membrane surfaces mainly include energy dispersive X-ray spectroscopy (EDS/EDX),
inductively coupled plasma (ICP), X-ray photoelectron spectroscopy (XPS), etc The morphology of active materials
and membrane surfaces is mainly observed by scanning electron microscopy (SEM),
transmission electron microscopy (TEM), etc For the crystal structure of active materials,
there are mainly techniques such as X-ray diffraction (XRD), neutron diffraction (ND),
nuclear magnetic resonance (NMR), etc The substances attached to the surface of the polarizer, separator,
and electrolyte mainly include Raman scattering spectroscopy (Raman),
Fourier transform infrared spectroscopy (FTIR), etc The main pathways for ion transport in materials include neutron diffraction (ND),
scanning tunneling microscopy (STM),
and atomic force microscopy (AFM). In addition, for the possible thermal runaway of lithium-ion batteries,
thermal performance tests of electrode active materials and separators need to be conducted using
thermogravimetric differential scanning calorimetry (TGA-DSC) or accelerated rate calorimetry (ARC);
For gas production, gas chromatography (GC) is required to analyze the composition of the gas;
For adhesive failure, it is necessary to analyze the relative molecular weight of the adhesive, understand its performance,
and test the residual electrolyte composition and the surface of the electrode and diaphragm.
In addition to directly testing the components,
it is also possible to disassemble and assemble the positive and negative electrode plates separately into button half cells,
and then test their electrochemical performance: gram capacity, impedance spectrum, and cyclic voltammetry curve.
Then, compared with the battery in a fresh state, combined with the characterization results of the active material,
analyze whether the failure of the battery (such as capacity decay, impedance increase, etc.) is mainly caused by the positive or negative electrode.
In addition, the separator can be assembled into a button type battery and compared with
a button type battery assembled with a brand new separator to test its impedance spectrum,
cyclic voltammetry curve, etc. to compare the performance changes of the separator.
The main testing methods for battery performance and component properties are shown in Table 6.
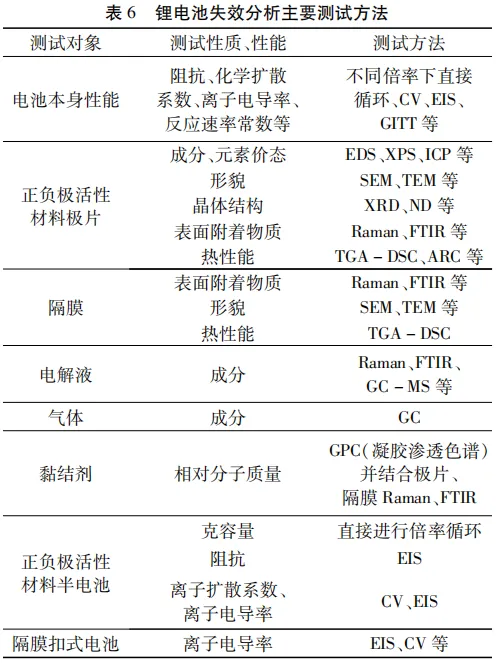
4 main reasons for lithium-ion battery failure
After analyzing the failure of lithium-ion batteries through the above process and testing methods,
the failure causes can be mainly divided into two categories:
one is the manufacturing defects of lithium-ion batteries themselves,
and the other is the external environment (including battery usage process, working environment, etc.).
Reasons for lithium-ion batteries themselves:
① Performance degradation, failure of positive and negative active materials, aging of separators,
electrolyte consumption, failure of binders, and corrosion of current collectors;
② Gas production, residual trace water during assembly, other active impurities in the slurry, corrosion of the current collector, etc;
③ Thermal runaway, unstable decomposition of positive electrode material produces oxygen and reacts with electrolyte to release heat,
and components such as separator and shell cannot dissipate heat quickly, resulting in heat accumulation;
④ Internal short circuit, direct contact caused by displacement of the current collector during assembly,
aging and failure of the diaphragm to insulate electrons, metal impurities in the slurry piercing the diaphragm,
and insufficient negative electrode capacity during design leading to the formation of lithium dendrites piercing the diaphragm.
External environmental factors:
① Performance degradation, operating temperature too high or too low, overcharging or overdischarging;
② Gas production, high working temperature, puncture;
③ Thermal runaway, high working current, high working temperature, external short circuit, puncture, overcharge and overdischarge;
④ Internal short circuit, overcharge and over discharge, puncture, and compression.
Even due to external environmental factors, the vast majority of battery failures are indirectly caused by reactions
and changes in the internal components of the battery.
Therefore, non-destructive testing of the battery itself
and damage detection of disassembled components are very important to fundamentally determine the cause of battery failure.






