Lithium batteries - Design - What is the typical surface capacity of a battery
Aug,08,24
On August 8, 2024 at 16:40, Jiangsu's surface capacity, as the core consideration factor in polarizer design,
refers to the storage capacity that can be carried per unit polarizer area,
which is generally quantified using measurement units such as mAh/cm ² or Ah/m ².
This parameter has a direct and significant impact on the energy density and power density of the battery.
Therefore, in the layout of the electrode plates, it is necessary to closely combine
the actual application scenario requirements of the battery
and finely regulate the surface capacity level to achieve the optimal balance of the battery's comprehensive performance.
The pursuit of higher surface capacity often requires the polar coating to bear higher loads,
resulting in a corresponding increase in polar thickness while maintaining the same compaction density.
This design strategy significantly increases the loading capacity of active materials by reducing the proportion of non active components in the battery,
which not only enhances the energy density of the battery but also helps optimize cost-effectiveness.
For example, when the electrode thickness jumps from 25 microns (approximately equivalent to 8 milligrams per square centimeter of active material load)
to 200 microns (approximately equivalent to 64 milligrams per square centimeter of active material load),
the proportion of non active components can sharply decrease from 44 weight percent to 12 weight percent,
and the amount of active material load increases by more than 30%,
which is intuitively reflected in the significant improvement of battery energy density (as shown in Figure 1a).
However, the increase in electrode thickness is also accompanied by challenges:
the transport path of charges (including electrons and ions) is prolonged, resulting in an increase in resistance and affecting the kinetic efficiency of charge transfer.
Specifically, lithium ions require longer time to reach and store at the active sites in thicker electrodes,
which limits the battery's rapid charge discharge capability, i.e. rate performance,
and may also pose a bottleneck for further improvement in energy density (as shown in Figure 1b).
In addition, during the manufacturing process, the drying stage of thick electrodes is prone to fracture and delamination,
which poses technical challenges for producing stable and reliable electrode structures (as shown in Figure 1c).
In summary, the design of electrode surface capacity (or thickness) needs to seek a balance point,
which is to ensure the maximization of battery energy density while avoiding performance degradation and manufacturing difficulty caused by increasing thickness.
The determination of this optimal range is the key to improving the overall performance of batteries and meeting diverse market demands (as shown in Figure 1b).
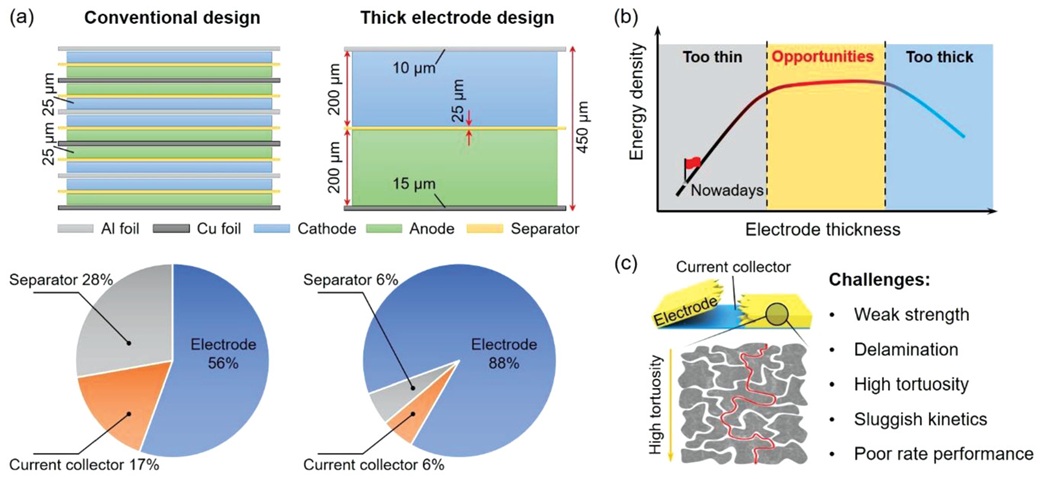
Figure 1 Opportunities and Challenges of Thick Electrode Design
What is the current level of surface capacity of electrode coating in the field of commercial batteries?
In order to gain insight into the practical application of this design parameter,
we have synthesized detailed analysis reports and disassembly data of commercial batteries,
and systematically summarized the surface capacity of the electrode plates.
When exploring this key indicator, there are two mainstream approaches for measuring and calculating facial features:
Direct disassembly testing method: This method involves finely disassembling commercial batteries, peeling off single-sided coated electrode pieces,
and cleverly reassembling these electrode pieces into small button batteries for testing.
By accurately measuring the capacity and corresponding electrode area, we can directly calculate the surface capacity.
It is worth noting that in this process, lower test magnifications are often used to ensure that
the measured surface capacity is closer to the theoretical expected value at the time of design,
thus reflecting a more realistic performance level.
Indirect capacity estimation method: Another method relies on the rated capacity of the battery
and the precise measurement of the electrode coating area after disassembly.
Firstly, the rated capacity of the battery obtained through official or actual testing is used as the starting point,
and then combined with the coating area information in the disassembly data, the surface capacity of the positive electrode plate is calculated.
For the negative electrode surface capacity,
it is usually estimated based on the industry practice of an N/P ratio (mass ratio of negative electrode to positive electrode active material) of 1.1.
However, this method may result in lower calculation results compared to direct testing methods due to various factors,
reflecting the difference between ideal conditions and actual use.
The following is a detailed summary of the surface capacity results obtained based on the above two methods,
presented in a clear table format for intuitive understanding and analysis of the current design status of the extreme surface capacity of commercial batteries.
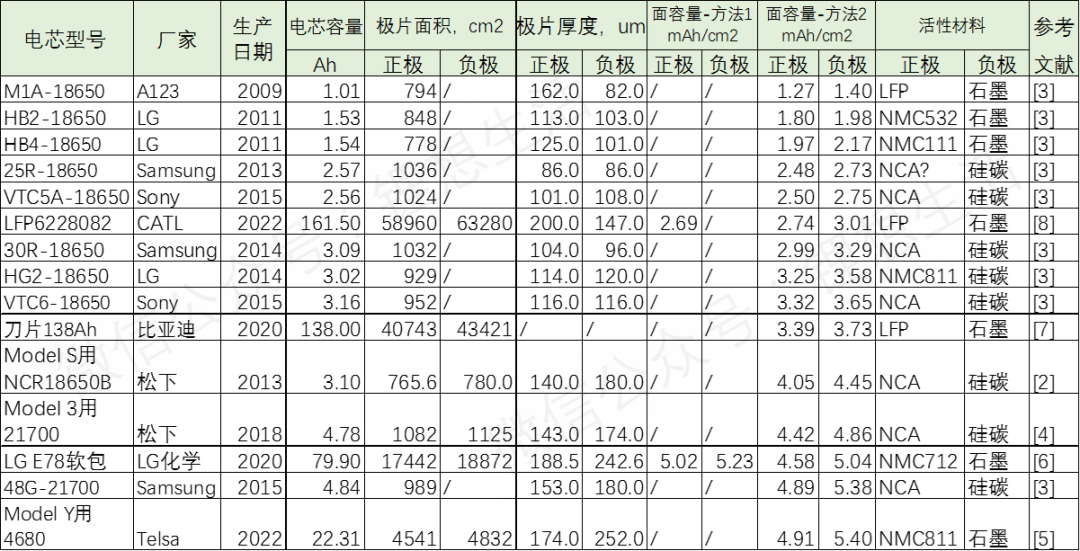
Figure 2 clearly illustrates the intrinsic relationship between the positive electrode surface capacity and the battery power to energy ratio.
This ratio intuitively reflects two key dimensions of battery performance: when the power to energy ratio of the battery tends to increase,
it indicates that its power characteristics are more superior, that is, the battery can charge and discharge faster;
On the contrary, if the ratio decreases, it means that the energy density of the battery is higher, that is, more energy is stored per unit volume or mass.
In the vast field of battery design, it can be mainly divided into two categories: power type batteries and energy type batteries.
Power type batteries stand out with their excellent rate performance, maintaining high efficiency and stability even under high-speed charging and discharging conditions.
However, this advantage often comes with a relative sacrifice in energy density.
To achieve this goal, power type batteries typically adopt a low surface capacity thin electrode design strategy to optimize the charge transfer path,
reduce internal resistance, and thus improve power response speed.
In contrast, energy batteries focus on increasing energy density
and are committed to storing more energy in limited space or weight to meet the demand for long battery life.
However, this characteristic often comes with a compromise in power characteristics.
To achieve high energy density, energy type batteries tend to adopt thick electrode designs with high surface capacity,
which store more energy by increasing the load of active materials.
However, this also increases the complexity and time cost of charge transfer, affecting the rapid charging and discharging ability of the battery.
In summary, the positive electrode capacity plays a crucial role in battery design as a bridge connecting the power and energy characteristics of the battery.
Its design choice directly determines whether the battery is biased towards power or energy based application scenarios.
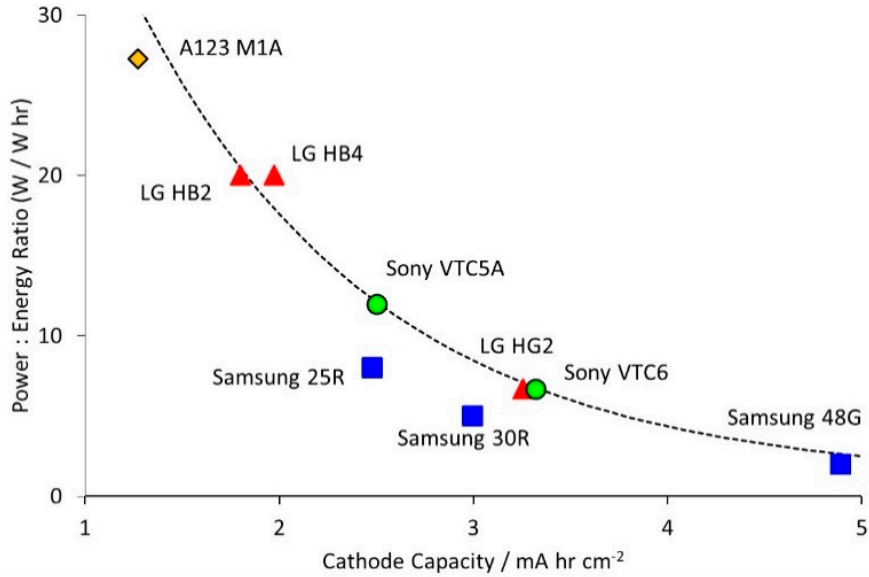
Figure 2 Relationship between positive electrode capacity and battery power to energy ratio
Among the mentioned battery arrays, A123's 18650 lithium iron phosphate battery, launched in 2009,
stands out with its extremely low surface capacity of only 1.27mAh/cm ², clearly demonstrating its positioning as a power battery.
This battery greatly reduces the resistance of lithium ions during transmission and diffusion by adopting an ultra-thin electrode design,
endowing it with extraordinary capabilities of high power output and ultra fast charging.
LG Chem's HB2 and HB4 series 18650 batteries also adopt the concept of thin polarizer design,
with a surface capacity maintained at a level below 2.0mAh/cm ², further confirming their typical representative position in the field of power batteries.
In contrast, Tesla's 4680 battery leads the industry with its outstanding extreme surface capacity design, reaching up to 4.91mAh/cm ²,
thanks to the dry electrode process used in the negative electrode.
This process cleverly bypasses the traditional wet coating and drying process's limitation on electrode thickness,
allowing the graphite negative electrode thickness to jump to over 250 microns, indicating that the potential surface capacity can approach 5.4mAh/cm ².
Although the high positive electrode capacity design and relatively thick battery casing and component design have not yet reached the ultimate energy density,
they undoubtedly leave ample room for future performance improvement.
In addition, Panasonic's 18650 and 21700 batteries tailored for Tesla,
as well as LG Chem's E78 soft pack battery and Samsung's 48G21700 battery, all demonstrate high surface capacity designs exceeding 4.0mAh/cm ²,
highlighting the outstanding pursuit of these products in high energy density and high performance.
As for most other battery products, their extreme capacity designs mostly fall within a relatively balanced range of 2.5 to 4.0mAh/cm ²,
taking into account both energy density and power characteristics, meeting the diverse application needs in the market.






