Lithium Battery - Knowledge - The Influence of Voltage on Gas Production in High Temperature Storage of Lithium Batteries
Aug,09,24
At present, there have been reports on the phenomenon of gas production in lithium-ion batteries,
but there are few systematic reports on the storage performance of lithium-ion batteries at 70 ℃ under different voltages
or the reduction gas production of negative electrode active materials during storage at 70 ℃ under different voltages.
During transportation, application, and storage,
lithium-ion batteries may encounter high temperature storage due to their complex and variable state of charge and environmental conditions.
This article studies the storage performance of square lithium-ion batteries with different voltages at 70 ℃, and conducts relevant tests on bulging failed batteries.
In depth analysis is conducted on the internal gas production composition, positive and negative electrode gas production, material structure changes, and other data.
1 Experiment
1.1 Battery and Materials
The experimental battery is a traditional square lithium battery with a rated capacity of 1.5Ah.
The positive electrode active material of the battery is lithium cobalt oxide, and the negative electrode active material is artificial graphite.
The diaphragm adopts 16um PE diaphragm.
1.2 Instruments and Testing
Perform cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) tests on the button cell (CR2430) using an electrochemical workstation.
Use battery charging and discharging equipment to test the charging and discharging curves of button batteries and square batteries.
Use an X-ray diffraction spectrometer to perform X-ray diffraction spectroscopy (XRD) to test the structural changes of negative electrode crystals at different voltages.
Use a gas chromatography-mass spectrometry (GC-MS) to test the gas composition.
The testing method for storage performance at 70 ℃ is as follows: test the thickness T1, voltage V1,
and internal resistance R1 of batteries with different voltages (~4.2,~3.9,~3.7,~3.5,~3.3,~3.2, and~3.0V) at room temperature,
and store the batteries in a high temperature box at 70 ℃ for 7 days.
Then remove the batteries and place them at room temperature for 2 hours to test the thickness T2, voltage V2,
and internal resistance R2 of the batteries, and calculate the expansion rate.
The experimental method for gas production analysis is as follows:
Select fresh batteries of different voltages (with the same voltage as above) and dissect them in a glove box.
Take out the positive and negative electrodes, and pack 5 3cm x 3cm sized positive and negative electrodes into aluminum-plastic bags.
Add 1mL of electrolyte, vacuum seal, and test the volume v1. Store the packaged polarizer in a high temperature box at 70 ℃ for 7 days,
then take it out and place it at room temperature for 2 hours to test the volume v2 and calculate the gas production Δ v.
2 Results and Discussion
2.1 Battery storage performance test at 70 ℃
Select batteries with different voltages (as above), first test the battery thickness T1, voltage V1, and internal resistance R1 at room temperature,
then store the battery in a 70 ℃ high-temperature box for 7 days,
and then remove the battery and place it at room temperature for 2 hours to test the battery thickness T2, voltage V2,
and internal resistance R2, and calculate the expansion rate.
The data on thickness expansion, voltage, and internal resistance changes caused by gas production from different batteries are shown in Table 1.
Table 1 Changes in battery thickness expansion rate, voltage, and internal resistance before and after storage testing at 70 ℃
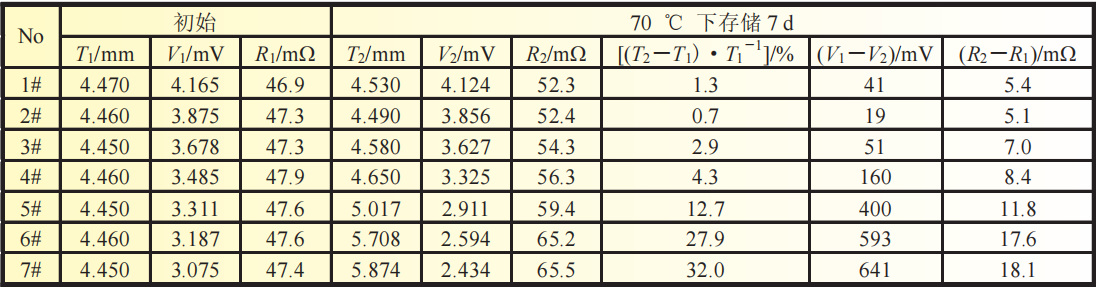
As shown in Table 1, after being stored at 70 ℃ for 7 days,
the voltage of each battery decreased, and the thickness and internal resistance of the battery increased.
As the voltage of batteries with different initial voltages (1 #~7 #) decreases,
the overall thickness expansion rate of the batteries shows an increasing trend. From the expansion rate data,
the increase in expansion rate of batteries 1 # to 4 # is not significant, all less than 5%.
The expansion rates of batteries 5 # to 7 # increased significantly, reaching 12.7%, 27.9%, and 32%, respectively.
Compared to the voltage drop data, the increase in voltage drop for batteries 1 # to 4 # is relatively small,
while the increase in voltage drop for batteries 5 # to 7 # is significant;
From the data of internal resistance growth, the increase in internal resistance of batteries 1 # to 4 # is relatively small,
while the increase in internal resistance of batteries 5 # to 7 # is significant.
Therefore, based on the above results analysis, it can be concluded that there is a significant change in the trend of each data between batteries 4 # and 5 #,
and batteries 5 # to 7 # show obvious failure, with voltage<3.3V being the main cause of battery failure.
2.2 Analysis of Gas Production from Positive and Negative Pole Storage at 70 ℃
Select batteries with different voltages (the same voltage as above),
dissect and remove the positive and negative electrodes from the glove box, package them separately, and test the volume v1.
Store the packaged polarizer in a high temperature box at 70 ℃ for 7 days,
then take it out and place it at room temperature for 2 hours to test the volume v2 and calculate the gas production Δ v.
The gas production data of the positive and negative electrodes stored at 70 ℃ are shown in Table 2.
Table 2 Test results of positive and negative electrode gas production before and after 70 ℃ storage test
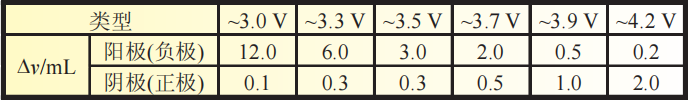
As shown in Table 2, after being stored at 70 ℃ for 7 days, both the positive and negative electrode plates produced gas.
According to the negative electrode gas production data, as the battery voltage decreases, the negative electrode gas production gradually increases,
especially when the voltage drops below~3.3V, the gas content increases significantly.
According to the positive electrode gas production data, as the battery voltage increases, the positive electrode gas production gradually increases.
When the voltage rises above~3.9V, the positive electrode gas production is more significant.
By comparing the gas production phenomena of the positive and negative electrodes,
it can be seen that the negative electrode produces significantly more gas in low voltage batteries than in high voltage batteries,
while the positive electrode produces significantly more gas in high voltage batteries than in low voltage batteries.
Additionally, an important piece of information is that after being stored at 70 ℃ for 7 days,
the gas production of the negative electrode is significantly higher than that of the positive electrode.
2.3 Analysis of Gas Generation Components in Square Batteries
In order to study the gas production mechanism of square batteries, this article tested the gas composition of the gas produced by square batteries.
Figure 1 shows the gas chromatography test results of the gas produced by a square battery (3.3V).
As shown in Figure 1, after being stored at 70 ℃ for 7 days, the gas components in the square battery are CO, CH4, CO2, C2H4, C2H6, C3H6, C3H8, etc.
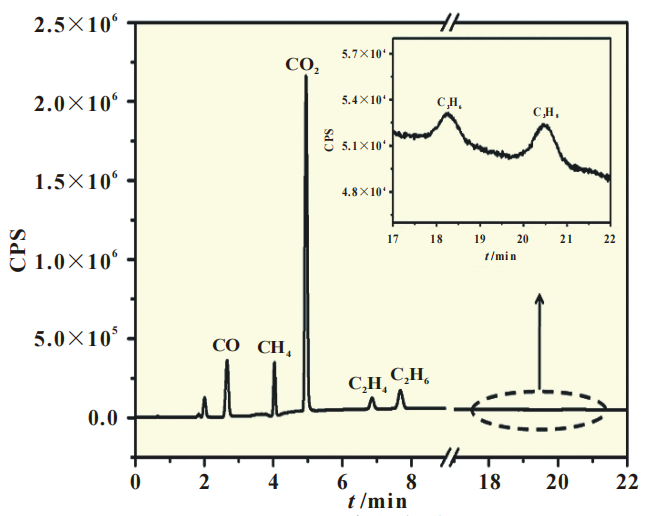
Figure 1 Gas chromatogram
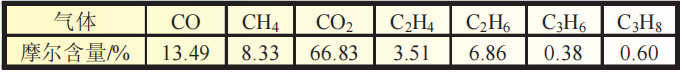
Table 3 shows the gas composition analysis results of the gas produced by the square battery after storage testing at 70 ℃.
As shown in Table 3, the gas produced by the battery has the highest CO2 content, and also contains CO, CH4, C2H4, C2H6, C3H6, and C3H8.
In the above gases, we speculate that CH4, C2H6, and C3H8 are reduction reaction products of the negative electrode side electrolyte,
while other gas components may be oxidation reaction products of the positive electrode side electrolyte.
Therefore, based solely on the above data, it is not sufficient to determine the specific reaction situation that occurs inside the square battery when stored at 70 ℃.
Table 3 Gas Composition Test of Square Battery Produced Gas after 70 ℃ Storage Test
2.4 Analysis of Gas Composition Produced by Positive and Negative Electrodes
In order to further determine the specific chemical reactions that occur inside the battery when stored at 70 ℃,
this article also conducted gas chromatography tests and component analysis on the gases produced by the positive
and negative electrodes of square batteries with different voltages.
Table 4 shows the test and analysis results of the gas composition of the positive and negative electrodes after storage at 70 ℃.
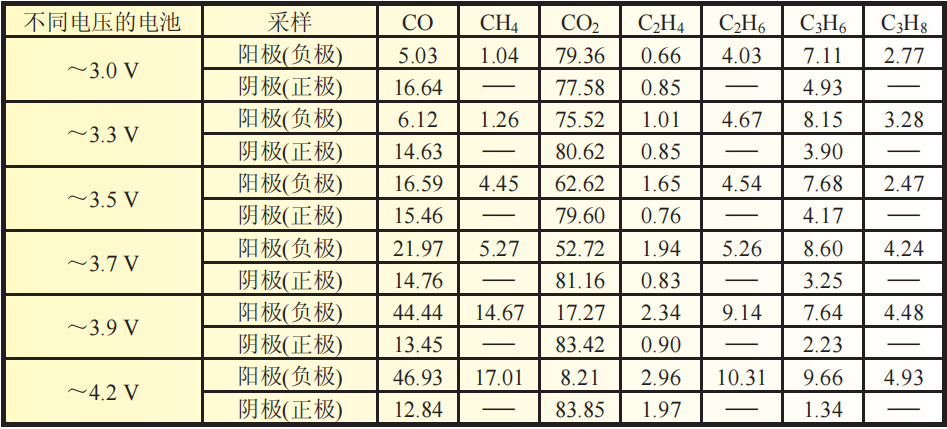
The electrolyte undergoes decomposition reaction on the negative electrode, and the gas composition of the gas produced is CO2, CO, CH4, C2H4, C2H6, C3H6, and C3H8.
The electrolyte undergoes a decomposition reaction on the positive electrode, and the gas components produced are CO2, CO, C2H4, and C3H6.
Table 4 Gas composition mole fraction test of positive and negative electrodes of batteries with different voltages stored at 70 ℃
Based on experimental results and literature analysis, we speculate that when stored at 70 ℃,
the gas production of the battery mainly includes the reduction and decomposition reaction of the negative electrode side electrolyte,
the oxidation and decomposition reaction of the positive electrode side electrolyte, and the decomposition reaction of the electrolyte under Lewis acid PF5.
Figure 2 shows a schematic diagram of the gas production mechanism during storage at 70 ℃.
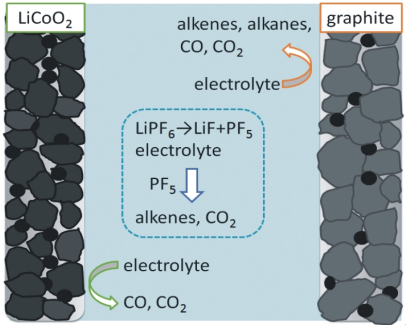
Figure 2 Schematic diagram of gas production mechanism in 70 ℃ storage
2.4.1 Reduction reaction of negative electrode electrolyte

2.4.2 Decomposition reaction of electrolyte on positive electrode
The gas composition of the produced gas contains CO2, CO, C2H4, and C3H6.
The composition of gas produced by the positive electrode has been studied.
The positive electrode stored independently in a soft bag decomposes at high temperatures to generate O2,
which then continues to oxidize the electrolyte, mainly producing CO2, CO, and trace organic gas molecules.
In this work, the positive electrode storage produced gas, and in addition to CO and CO2, C2H4 and C3H6 were also detected.
We believe that the above-mentioned olefins may also be generated by the high-temperature decomposition reaction of the electrolyte,
and the analysis of the electro-hydraulic decomposition mechanism is described in section (2.4.3) below.
2.4.3 Lithium salt LiPF6 in electrolyte will undergo decomposition reaction at high temperature or even room temperature
The decomposition reaction is as follows:
LiPF6→LiF+PF5
PF5 is a strong Lewis acid, and the electrolyte undergoes the following reaction under the catalysis of the Lewis acid:
ROCO2R→ROR, R-F, alkenes, CO2
In fact, in the EC/PC/DEC/EMC electrolyte system used in this study,
in addition to CO and CO2 obtained from the oxidation reaction of the electrolyte on the positive electrode, olefin gas components
such as C2H4 and C3H6 were also detected in the gas composition obtained from the electrolyte reaction on the positive electrode side.
In summary, during the high-temperature storage process of the positive electrode,
olefins such as C2H4 and C3H6 are produced by the high-temperature decomposition reaction of the electrolyte.
In order to gain a more intuitive understanding of the reaction mechanism of the positive and negative electrode electrolytes,
the composition of gas produced by the positive and negative electrodes at different voltages is displayed in the form of a histogram.
As shown in Figure 3 (a), for the negative electrode, the content of CO and CO2 changes significantly with the increase of battery voltage.
As the battery voltage increases, the content of CO in the gas produced on the negative electrode side gradually increases,
and the increase is significant, from 5.03% to 46.93%;
The content of CO2 decreases sequentially, and the decrease is significant, from 79.36% to 8.21%.
The changes in CO and CO2 content indicate that different levels of lithium insertion in the negative electrode affect the pathway of electrolyte decomposition reaction.
In addition, the consumption of CO2 during the reaction with lithium embedded graphite (LiC)
on the negative electrode may also be one of the reasons why the CO2 content decreases
with the increase of battery voltage (the degree of lithium insertion in the negative electrode).
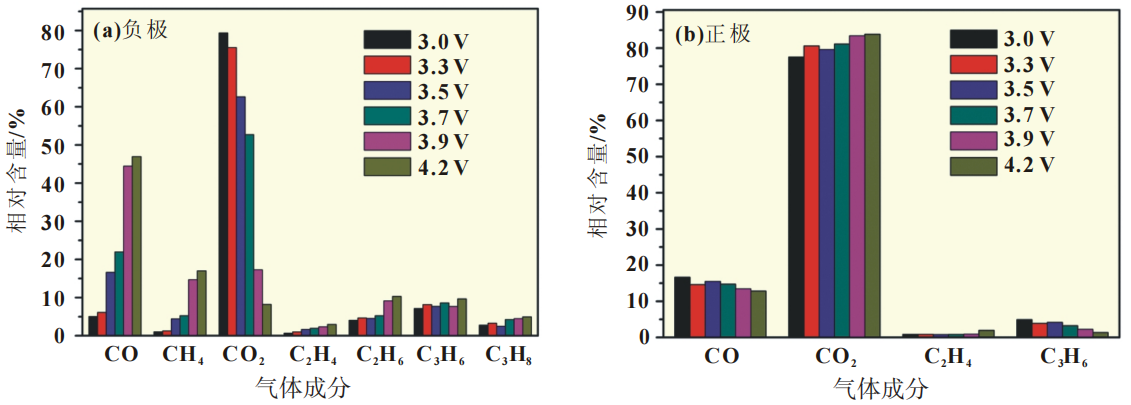
Figure 3 Gas composition after storage at 70 ℃
Unlike the analysis results on the negative electrode side,
the changes in the content of other components on the positive electrode side are not significant.
As shown in Figure 3 (b), for the positive electrode, with the increase of battery voltage,
the CO content in the gas composition on the positive electrode side slightly decreases,
while the CO2 content slightly increases.
The results indicate that there is no essential difference in the decomposition mechanism of the positive electrode side electrolyte with changes in battery voltage.
In addition, it has been confirmed that the consumption of CO2 in the battery mainly occurs on the negative electrode side.
2.5 Analysis of lithium insertion degree in positive and negative electrode materials
In order to further understand the influence of the degree of lithium insertion in the negative electrode
on the decomposition reaction of the electrolyte on the negative electrode side,
XRD testing and analysis were conducted on the negative electrodes of batteries with different voltages.
According to Bragg's formula 2dsin Θ=λ, the interlayer spacing d002 of graphite negative electrode material can be obtained.
According to Bragg's formula, the interlayer spacing of graphite is inversely proportional to Θ,
and as Θ shifts to the right, the interlayer spacing of graphite decreases.
As shown in Figure 4 (a) and Figure 4 (b), the evolution of the (002) and (004) peak positions of graphite with the variation of battery voltage is shown.
The peaks of (002) and (004) shift to the right as the voltage decreases.
When the voltage drops below 3.3V, the peak position does not change significantly,
indicating that the active lithium in the graphite negative electrode has completely desorbed when the battery voltage is 3.3V.
So, when discharging below 3.3V voltage, the negative electrode of the battery will undergo SEI film oxidation and decomposition reaction.
Based on the above analysis results, we believe that the increase in gas production on the negative electrode side
with the decrease of battery voltage may be caused by two reasons:
(1) as the battery voltage decreases, the degree of lithium insertion in the negative electrode decreases, the stability of the SEI film decreases,
and the secondary reaction between the SEI film and the electrolyte increases;
(2) As the battery voltage decreases, the degree of lithium insertion in the negative electrode decreases,
the amount of lithium insertion in graphite decreases, and the consumption of CO2 caused by the reaction with CO2 decreases.
3 Conclusion
This article studies the high-temperature gas production mechanism of batteries.
Through the analysis of gas production and gas composition of positive and negative electrode plates of batteries with different voltages stored at 70 ℃,
it is proved that the gas production of lithium-ion batteries stored at 70 ℃ is mainly caused by
the reaction between the SEI film oxidation and decomposition of the negative electrode and the electrolyte after complete lithium removal.
The change in gas production is related to the degree of lithium insertion in the negative electrode.
The experimental results show that when stored in a high temperature environment of 70 ℃,
the gas production of the positive electrode is less than that of the negative electrode,
while the gas production of the negative electrode in low-voltage batteries is much higher than that of high-voltage batteries.
The above experimental results can also guide us in voltage control during battery transportation, storage, and operation.
In addition, they have significant guiding significance for the design of battery electrolytes.






