A new strategy for preparing ultra-thin metallic lithium!
Aug,15,24
【 Research Background 】
Ultra thin metallic lithium is the key to achieving high energy density in lithium metal batteries,
but the low-cost mass production of high-performance ultra-thin metallic lithium negative electrodes is
a bottleneck hindering the commercialization of lithium metal batteries.
The industrial production of commercial lithium foil is mainly achieved through extrusion, rolling, and surface passivation.
Thin metal lithium foil with a thickness of 20-100 μ m can be prepared by multi roll rolling,
but due to the high viscosity and poor mechanical processing performance of metal lithium
at room temperature, it is difficult to obtain thinner thicknesses.
In addition, methods such as electrodeposition or physical vapor deposition
can accurately adjust the thickness of ultra-thin lithium foil,
but they are time-consuming and require bulky vacuum chambers for physical vapor deposition.
In recent years, many new methods have been reported for the preparation of ultra-thin lithium,
such as molten lithium alloy "ink paste", lithium friendly layer coating to improve molten lithium infiltration,
construction of three-dimensional nano material skeleton, lithium powder slurry coating,
chemical reagent etching, magnetic driven coating of molten lithium, and rolling.
Although many methods have been attempted to prepare ultra-thin lithium,
the high performance of ultra-thin metal lithium negative electrodes often depends on cumbersome,
expensive, and laboratory scale preparation methods.
Unfortunately, the development of feasible, large-scale,
and low-cost methods for preparing high-performance ultra-thin lithium metals remains a bottleneck.
[Job Introduction]
Recently, Professor Li Jingze's research group at the University of Electronic Science and Technology of China proposed
a simple method for preparing ultra-thin metal lithium foil by directly coating molten lithium liquid onto heated copper foil roll to roll.
The author points out that suitable temperature conditions can accelerate
the alloying reaction between molten lithium and copper foil,
greatly improving the wettability between molten lithium and copper foil,
allowing molten lithium to quickly infiltrate the surface of copper foil,
achieving the effect of uniformly spreading and preparing ultra-thin lithium foil.
The achievement was published in Energy Storage Materials under the title "Endowing Cu Coil Self Wettable in Molten Lithium:
A Roll to Roll Wet Coating Strategy to Fabricate High Performance Ultrathin Lithium Metal Anodes".
Xing Jianxiong is the first author of this article.
[Content Description]
Due to its excellent conductivity, high structural/electrochemical stability, and low cost,
copper foil is commonly used as a negative electrode current collector for lithium-ion or lithium metal batteries.
Meanwhile, there is a significant difference in melting points between metallic lithium (180.5 ℃) and metallic copper (1083.4 ℃).
Therefore, it is feasible to prepare ultra-thin lithium metal by coating the surface of copper foil with molten lithium liquid metal.
However, the wettability of molten lithium on unheated copper foil is poor,
making it difficult to evenly spread the molten lithium on the surface of the copper foil.
By modifying the surface of copper foil with some lithium friendly media (such as organic compounds, metal oxides, and alloying metals),
the wettability of copper foil can be effectively improved.
However, the additional lithium friendly surface modification increases the preparation cost and limits its commercial practicality.
In addition, reports have shown that copper metal can undergo alloying reactions
with molten lithium at high temperatures and form LiCux solid solutions with higher lithium affinity.
Therefore, it is possible to improve the affinity between molten lithium and copper foil by in-situ lithium copper alloying,
thereby hot-melt coating molten lithium onto heated copper foil.
In addition, the solidified LiCux solid solution forms a three-dimensional network skeleton structure inside ultra-thin metallic lithium due to segregation,
which can effectively reduce local current density and suppress the growth of lithium dendrites,
promote uniform lithium deposition process, and achieve high-performance ultra-thin metallic lithium.
In order to investigate the effect of temperature on the wettability of molten lithium on copper foil surfaces,
the authors investigated the different forms of molten lithium on copper foil surfaces between 200 ℃ and 475 ℃.
It was found that under temperature conditions below 250 ℃, molten lithium exhibits poor wettability towards copper foil;
When the temperature exceeds 275 ℃, molten lithium can exhibit good wettability on copper foil,
and the contact angle of molten lithium on the surface of copper foil significantly decreases;
When the temperature exceeds 350 ℃, although molten lithium exhibits excellent wettability on copper foil,
due to the rapid alloying reaction between molten lithium and copper foil, copper foil is easily dissolved in molten lithium.
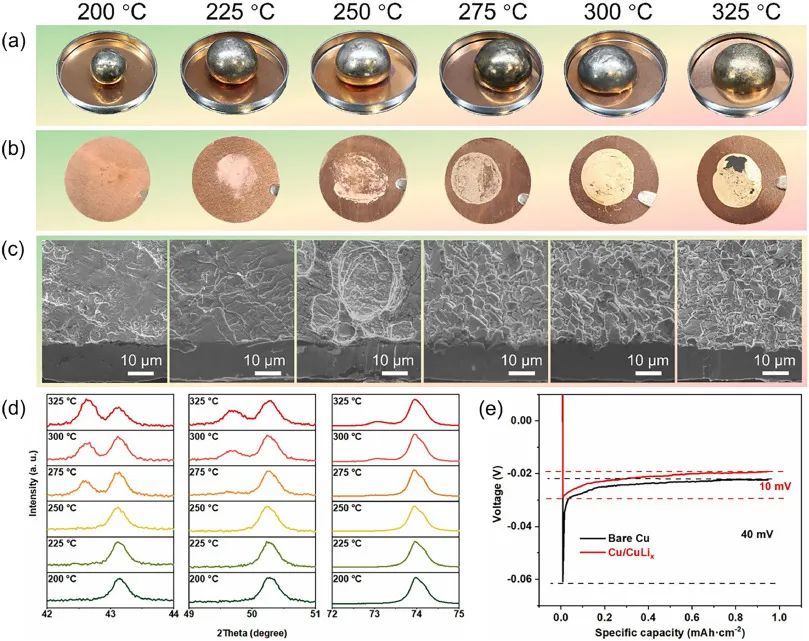
Figure 1: (a) Optical photographs of the surface morphology of molten lithium on copper foil under different temperature conditions (200-325 ℃);
(b) Optical photograph of copper foil surface after molten lithium etching;
(c) Corresponding etched copper foil tilted surface cross-sectional SEM image;
(d) XRD patterns of etched copper foil in the selected 2 θ (42-44 °, 49-51 °, and 72-75 °) range;
(e) Capacity voltage curves of bare copper foil and LiCux (etched copper foil) electrodes
Therefore, by precisely controlling the temperature of copper foil and molten lithium,
an ultra-thin layer of metallic lithium can be scraped onto the surface of copper foil using direct hot melt coating.
Due to the slight dissolution of copper foil, a large amount of LiCux solid solution three-dimensional skeleton structure is formed by copper segregation inside,
and the resulting ultra-thin lithium is named ultra-thin Li/LiCux.
The lithium layer thickness of ultra-thin Li/LiCux based on copper current collector can be controlled between 5-50 μ m,
with a width of 10 cm and a length of at least 50 cm, which can basically meet the requirements of negative electrode materials for metal lithium batteries.
And due to its composite with copper foil, ultra-thin Li/LiCux has excellent flexibility and mechanical strength, without any damage when folded or bent,
which is beneficial for production and transportation.
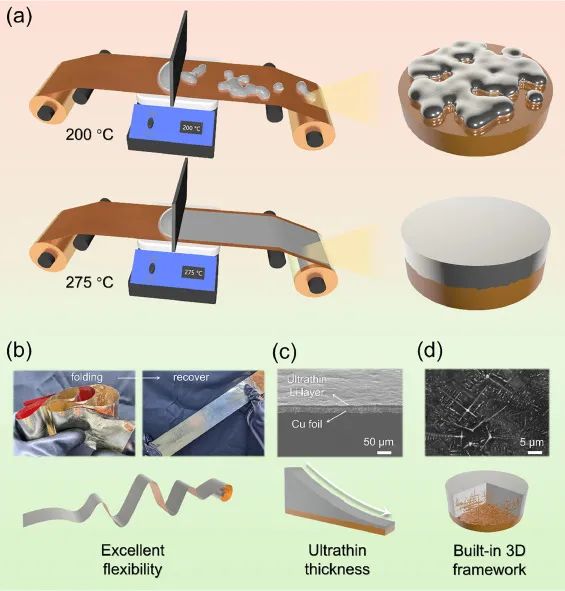
Figure 2: (a) Schematic diagram of preparing ultra-thin lithium under different temperature conditions;
(b) Flexibility testing of ultra-thin LiCux/Li; (c) Cross section and (d) surface SEM images of ultra-thin LiCux/Li
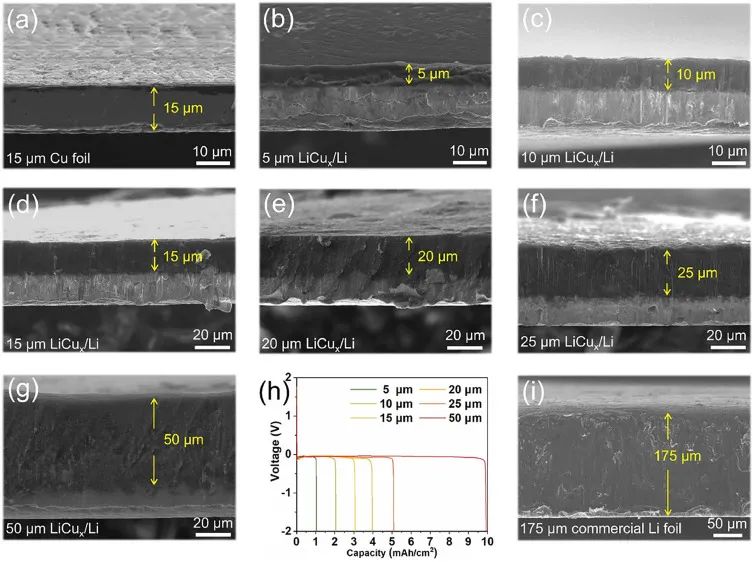
Figure 3: (a) Cross sectional SEM images of ultra-thin LiCux/Li with original copper foil and thicknesses of (b) 5 μ m,
(c) 10 μ m, (d) 15 μ m, (e) 20 μ m, (f) 25 μ m, and (g) 50 μ m, respectively;
(h) Lithium stripping curves of ultra-thin LiCux/Li electrodes with different thicknesses;
(i) Cross sectional SEM image of 175 μ m thick commercial lithium foil
To evaluate the electrochemical performance of ultra-thin LiCux/Li composite negative electrode,
the authors assembled a symmetrical battery and used pure ultra-thin metallic lithium as a control.
When using a DOL/DME solution with 1 M LiTFSI dissolved (containing 2% LiNO3 additive) as the electrolyte,
symmetric cells assembled with ultra-thin LiCux/Li composite electrodes with a thickness of 25 μ m exhibited excellent
long-term cycling performance under 1 mA cm-2 and 1 mAh cm-2 conditions,
maintaining a stable polarization cycle of 13 mV for over 1200 hours.
In contrast, a symmetrical battery made of pure metallic lithium with a thickness of 25 μ m exhibits significant polarization (20 mV),
which gradually increases after only 300 hours. When using EC/DEC (containing 5% FEC) with 1 M LiPF6 dissolved as the electrolyte,
the ultra-thin LiCux/Li composite electrode with a thickness of 25 μ m can stably cycle for more than 300 hours.
The symmetrical battery with 25 μ m thick pure metallic lithium begins to increase polarization after 100 hours of cycling.
Moreover, after one or more cycles, the ultra-thin LiCux/Li electrode exhibits relatively smaller RSEI and Rct values compared to pure metallic lithium.
The ultra-thin LiCux/Li electrode calculated based on the Tafel curve also exhibits a higher exchange current density than the pure metal lithium electrode.
The above results indicate that under the same electrode thickness and different electrolyte environments,
the ultra-thin LiCux/Li electrodes prepared in this study exhibit much better electrochemical performance than pure metallic lithium.
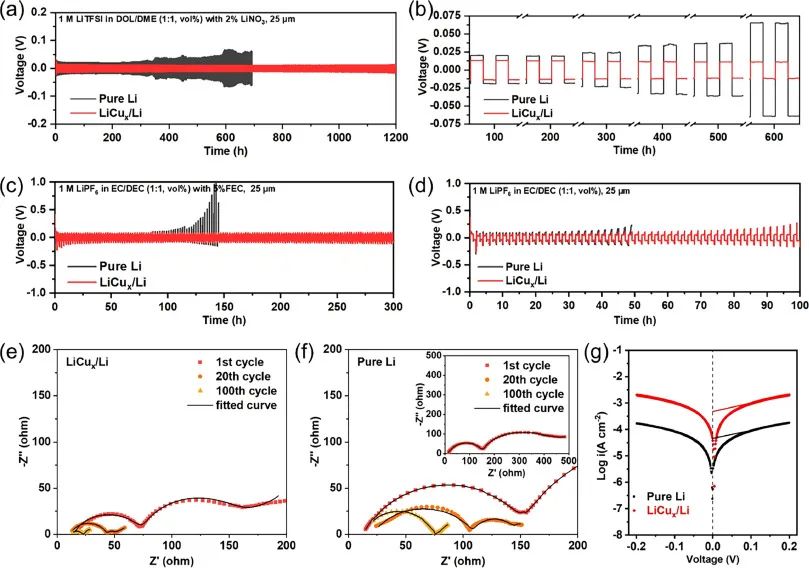
Figure 4: Charge discharge curves of ultra-thin LiCux/Li and pure Li symmetric batteries in different electrolytes under 1 mA-cm-2 and 1 mAh cm-2 conditions: (a-d);
(e) Nyquist plots of ultra-thin LiCux/Li and (f) ultra-thin pure Li symmetric batteries after different cycles at a test temperature of 25 ℃;
(g) Tafel diagram of symmetric batteries containing ultra-thin lithium and LiCux/Li
In order to evaluate the long-term cycling performance of ultra-thin LiCux/Li composite electrode as a negative electrode material in actual battery operation,
the authors used LFP as the positive electrode material, ultra-thin LiCux/Li composite electrode or ultra-thin pure metallic lithium as the negative electrode material,
and 1 M LiTFSI ether electrolyte containing 2% LiNO3 additive. Under different N/P ratio conditions,
the long-term cycling decay rate of active lithium in the entire battery was explored.
Two LFP cathodes with different surface capacities (1.58 and 2.45 mAh · cm − 2, respectively) and ultra-thin metallic lithium
with different thicknesses were matched to adjust the N/P ratio.
Using a 20 μ m thick ultra-thin LiCux/Li composite electrode as the negative electrode material and a 2.45 mAh · cm − 2 LFP as the positive electrode material,
the assembled full battery has an N/P ratio as low as 1.63 (LFP: 2.45 mAh · cm − 2; lithium: 4 mAh · cm − 2).
The battery can stably cycle for more than 220 weeks at a rate of 1 C,
while the LFP full battery using 20 μ m thick pure metallic lithium as the negative electrode material experiences
a rapid consumption rate of active lithium due to uneven lithium deposition/stripping behavior,
resulting in a sudden decrease in capacity after only 100 cycles.
When the N/P ratio is increased to 2.04 (LFP: 2.45 mAh · cm − 2; lithium: 5 mAh · cm − 2) and the negative electrode thickness is 25 μ m,
the ultra-thin LiCux/Li | | LFP battery maintains a stable high discharge capacity (128.1 mAh · g − 1) after 260 cycles,
while the discharge capacity of the Li | | LFP battery begins to sharply decrease after about 150 cycles.
At an N/P ratio of 2.53 (LFP: 1.58 mAh · cm − 2; lithium: 4 mAh · cm − 2),
the initial and 380 cycle discharge capacities of the ultra-thin LiCux/Li | LFP battery were 132.3 mAh · g − 1 and 110.6 mAh · g − 1, respectively, with a capacity retention rate of 83.6%.
In contrast, full batteries using ultra-thin pure metal lithium anodes showed a sharp decline after only 200 cycles.
When the N/P ratio is 3.16 (LFP: 1.58 mAh · cm − 2; lithium: 5 mAh · cm − 2),
under 1 C conditions, the ultra-thin LiCux/Li | | LFP battery can stably cycle for more than 480 cycles, maintaining 83.4% of its initial capacity.
The Li | | LFP battery experiences a sudden decrease in capacity after only 250 cycles.
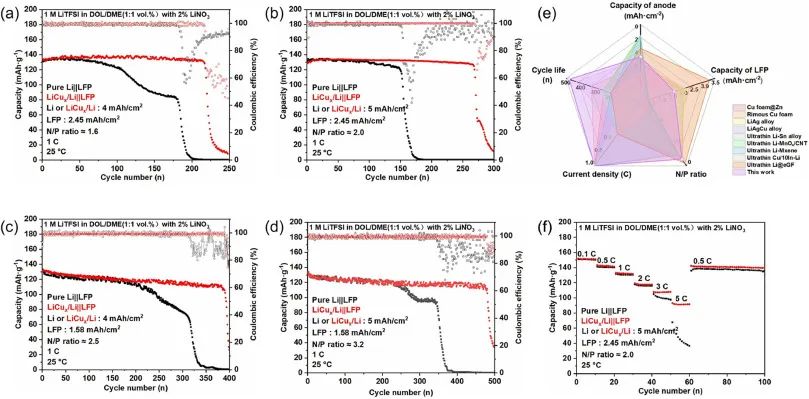
Figure 5: The cycling stability of ultra-thin Li | | LFP and LiCux/Li | | LFP full cells under different N/P ratios
at 1 C, with N/P ratios of (a) 1.6, (b) 2.0, (c) 2.5, and (d) 3.2, respectively;
(e) Comparison of electrochemical performance between ultra-thin LiCux/Li assembled full cell and related literature reports;
(f) Rate performance of LiCux/Li | | LFP and Li | | LFP full batteries
【 Conclusion 】
This study systematically investigated the effect of temperature on the wettability between molten lithium and copper foil,
and improved the wettability of molten lithium by regulating the temperature to promote in-situ alloying reaction between hot copper foil and molten lithium.
A roll to roll wet coating method for preparing ultra-thin metallic lithium was developed, resulting in ultra-thin lithium metallic lithium with a thickness of 5-50 μ m,
a width of 10 cm, and a length of at least 50 cm.
Therefore, without any pre modification operation, ultra-thin lithium foil materials can be prepared on a large scale, conveniently, and at low cost.
Moreover, a three-dimensional framework of LiCux solid solution alloy is formed in ultra-thin lithium,
which can reduce local current density and effectively suppress the formation of lithium dendrites,
thereby achieving uniform lithium stripping/deposition behavior and excellent electrochemical performance.
Under the conditions of 1 mA-cm-2 and 1 mAh cm-2, symmetrical batteries assembled based on ultra-thin LiCux/Li electrodes
with a thickness of 25 μ m exhibited cycling ability of over 1200 hours.
The full battery with an N/P ratio of 3.2, matched with a 1.58 mAh cm-2 LFP positive electrode, exhibits excellent long cycle capability,
maintaining a discharge specific capacity of 83.4% even after 480 cycles at 1 C.
This work provides a direct and effective method for the continuous production of ultra-thin lithium metal anodes,
promoting the commercialization process of lithium metal anodes.






