Cost analysis of power batteries for new energy vehicles
Aug,01,24
Virtual Capitalist website releases infographic analyzing the cost of electric vehicle power batteries
As the prices of electric vehicle (EV) batteries continue to decline, the global supply of electric vehicles and demand for their batteries are both increasing.
Since 2010, the average price of lithium-ion electric vehicle battery packs has decreased from $1200/kWh to $132/kWh.
Each electric vehicle battery pack contains multiple interconnected modules, consisting of dozens to hundreds of rechargeable lithium-ion batteries.
Overall, these batteries account for approximately 77% of the total cost of an average battery pack, at around $101 per kilowatt hour.
The percentage of each component of an electric vehicle battery to the total cost of the battery pack:
Cathode 51%
Manufacturing and depreciation 24%
Anode 12%
Separators 7%
Electrolyte 4%
Other materials 3%
Why are cathodes so expensive?
The cathode is the positive electrode of a battery.
When the battery is discharged, electrons and positively charged molecules (also known as lithium ions) flow from the anode to the cathode,
which stores both until the battery is recharged.
This means that the cathode effectively determines the performance, range, and thermal safety of the battery,
thereby determining the electric vehicle itself and making it one of the most important components.
They are composed of various metals (refined forms), depending on battery chemistry, typically including lithium and nickel.
The commonly used cathode components in modern times include:
Lithium iron phosphate (LFP)
Lithium nickel manganese cobalt (NMC)
Lithium nickel cobalt aluminum oxide (NCA)
The demand for battery metals that make up cathodes is high, and car manufacturers such as Tesla are competing to ensure supply.
In fact, the metal materials in the cathode and other parts of the battery account for about 40% of the total cost of the battery.
Components other than cathodes account for 49% of battery costs
he battery manufacturing process includes the production of electrodes, assembly, and completion of the battery, accounting for 24% of the total cost.
The anode is another important component of the battery, accounting for 12% of the total cost and about a quarter of the cathode.
The anode of lithium-ion batteries is usually made of natural or synthetic graphite, which is often cheaper than other metal materials.
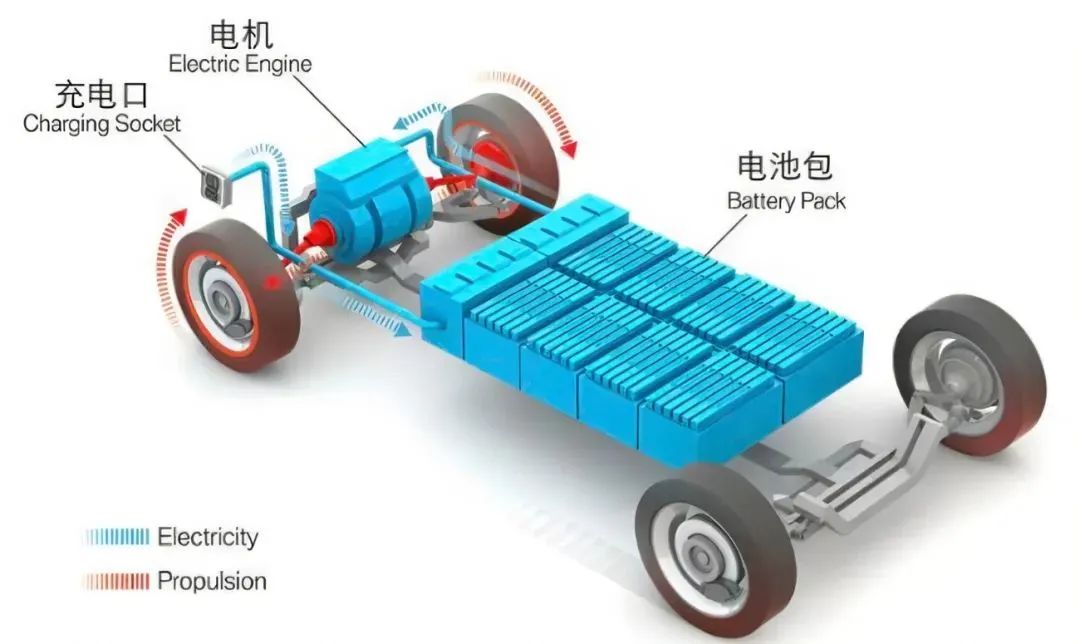
The Heart of New Energy Vehicles - Power Battery
As the power source of new energy vehicles, the power battery is the most important system in the entire vehicle, accounting for 30% to 40% of the vehicle cost.
This is also a distinctive component that distinguishes it from other traditional fuel vehicles.
The heart of traditional fuel vehicles is the engine, while the heart of new energy vehicles is the power battery.
At present, due to occasional safety accidents of new energy vehicles and severe reduction of winter range,
there are doubts about the future development prospects of new energy vehicles.
The main reasons are four: the range of new energy vehicles, the safety and convenience of power battery charging, and battery recycling.
And these four questions can be summarized as one problem: the issue of power batteries.
So, whether new energy vehicles are policy driven products or products that can replace fuel vehicles
and meet the real market demand in the future depends on whether they can solve the problem of power batteries?
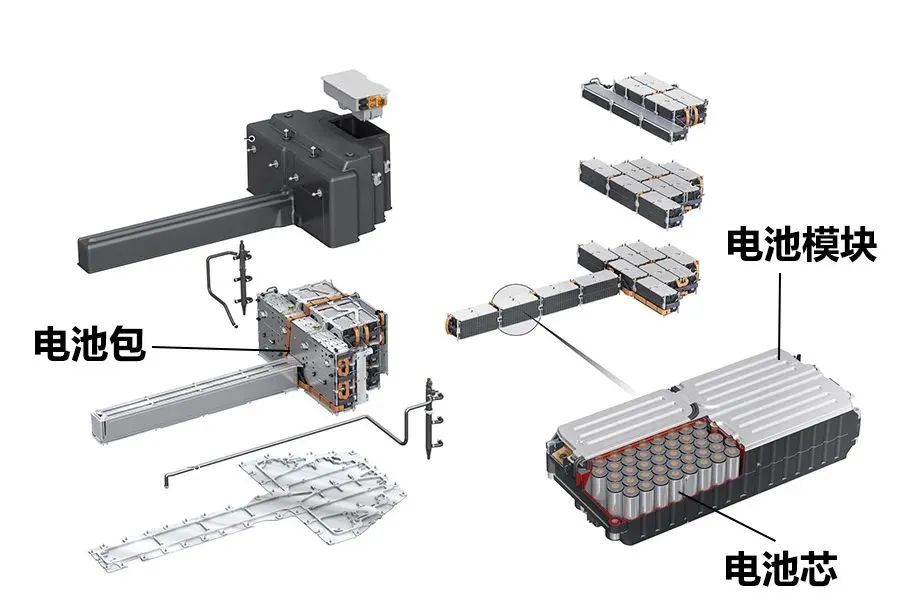
The structural composition of power batteries
The power battery is composed of several battery cells, CSC information acquisition system,
battery management control unit (BMU), battery high-voltage distribution unit, cooling system, etc.
Automotive power battery unit
A battery cell is the smallest unit that constitutes a storage battery, consisting of a positive electrode, a negative electrode, an organic electrolyte, etc.
The battery module is composed of several battery cells connected in parallel.
By connecting several battery packs in series to form a battery unit, and then connecting several battery units in series, a power battery assembly can be formed.
At present, the installed capacity of power batteries on the market is mainly provided by ternary lithium batteries and lithium iron phosphate batteries,
so below we will focus on explaining these two types of power batteries.
Power battery type - ternary lithium battery
Ternary polymer lithium battery, abbreviated as ternary lithium battery,
refers to lithium batteries with ternary positive electrode materials using nickel cobalt manganese oxide lithium (Li (NiCoMn) O2) or nickel cobalt aluminum oxide lithium.
The ternary composite positive electrode material is made of nickel salt, cobalt salt, and manganese salt as raw materials, and the ratio of nickel, cobalt,
and manganese can be adjusted according to actual needs.
Batteries with ternary materials as positive electrodes have higher safety compared to lithium cobalt oxide batteries.
Ternary lithium batteries are energy storage devices that integrate high energy density and high voltage,
and have been widely used in mobile and wireless electronic devices, power tools, hybrid power, and electric vehicles.
The reason why ternary lithium batteries are favored by many car companies is mainly due to the high energy density of ternary lithium batteries.
The higher the energy density, the more electricity can be stored per unit volume or weight of the power battery.
Generally speaking, the higher the energy density of the battery, the higher the range of pure electric vehicles.
Therefore, for new energy vehicle companies that strive for long range, the range advantage of ternary lithium batteries is very attractive.
At the same time, ternary lithium batteries also have certain advantages in low temperature resistance.
Under the same low temperature conditions, compared to other types of batteries,
ternary lithium batteries have smaller winter power attenuation and are more suitable for northern regions in cold winter.
The disadvantage of ternary lithium batteries is poor stability.
When the temperature reaches 250-350 ℃, they are prone to thermal runaway, and there is a high risk of spontaneous combustion during rapid charging.
Therefore, ternary lithium batteries have strict requirements for heat dissipation performance, which also has higher technical requirements for BMS battery management systems.
Power battery type - lithium iron phosphate battery
Lithium iron phosphate battery is a lithium-ion battery that uses lithium iron phosphate (LiFePO4) as the positive electrode material and carbon as the negative electrode material.
The rated voltage of a single cell is 3.2V, and its biggest advantage is high safety.
At present, the thermal stability of lithium iron phosphate batteries is the best,
and the temperature of thermal runaway is generally above 500 degrees, with a low risk of battery self ignition.
Secondly, the cycle life of lithium iron phosphate batteries is also relatively long, and they only begin to decay after more than 3500 charge and discharge cycles,
which is equivalent to being used for up to 10 years. In addition, lithium iron phosphate batteries also have a significant price advantage.
Lithium iron phosphate batteries have the advantages of high operating voltage, high energy density, long cycle life, good safety performance, low self discharge rate, and no memory effect.
However, due to the lower energy density of lithium iron phosphate batteries compared to ternary lithium batteries,
the average energy density of the former is 130-140Wh/kg, while that of ternary lithium batteries is 160Wh/kg.
Therefore, it is difficult to compare with ternary lithium batteries in terms of range, which is also why there are few pure electric vehicles using lithium iron phosphate batteries.
Power battery type - hydrogen fuel cell
Compared to batteries, hydrogen fuel cells, which are currently very niche, are truly "zero emission" clean energy sources.
They are power generation devices that directly convert the chemical energy of hydrogen and oxygen into electrical energy.
The basic principle is the reverse reaction of electrolyzing water, supplying hydrogen and oxygen to the cathode and anode respectively.
Hydrogen diffuses outward through the cathode and reacts with the electrolyte, releasing electrons through an external load to reach the anode, producing only water and heat.
It can be said that the advantages of hydrogen fuel cells are not only reflected in their high energy conversion efficiency, but also in their pollution-free and noise free nature.
From a long-term perspective, hydrogen fuel cells will undoubtedly be a key development direction for the future power battery industry.
For example, recently Hyundai announced a "2025 strategy", which not only aims to expand sales of pure electric vehicles, but also includes hydrogen fuel electric vehicles in its sales plan.
In addition, well-known car companies such as Toyota and Honda are actively promoting the development of hydrogen fuel cell technology.
However, at present, many problems with hydrogen fuel electric vehicles still cannot be effectively solved, mainly due to inconvenient hydrogen storage and high current cost.
The future development trend of power batteries is: higher energy density, faster charging speed, stronger safety, and lower cost.






