PRODUCT FEATURES
Cesium metal 99.95%/99.99%
Applicants :
* Petroleum exploration
The largest present-day use of nonradioactive caesium is in caesium formate drilling fluids for the extractive oil industry.
* Atomic clocks
A room with a black box in the foreground and six control cabinets with space for five to six racks each.
Electric power and electronics
Caesium vapour thermionic generators are low-power devices that convert heat energy to electrical energy.
Caesium is also important for its photoemissive properties, converting light to electron flow. It is used in photoelectric cells because caesium-based cathodes, such as the intermetallic compound K
CsSb, have a low threshold voltage for emission of electrons.The range of photoemissive devices using caesium include optical character recognition devices, photomultiplier tubes, and video camera tubes.
Caesium iodide (CsI), bromide (CsBr) and fluoride (CsF) crystals are employed for scintillators in scintillation counters widely used in mineral exploration and particle physics research to detect gamma and X-ray radiation. Being a heavy element, caesium provides good stopping power with better detection. Caesium compounds may provide a faster response (CsF) and be less hygroscopic (CsI).
Caesium vapour is used in many common magnetometers.
The element is used as an internal standard in spectrophotometry.Like other alkali metals, caesium has a great affinity for oxygen and is used as a "getter" in vacuum tubes.Other uses of the metal include high-energy lasers, vapour glow lamps, and vapour rectifiers.
Centrifugation fluids
The high density of the caesium ion makes solutions of caesium chloride, caesium sulfate, and caesium trifluoroacetate. useful in molecular biology for density gradient ultracentrifugation. This technology is used primarily in the isolation of viral particles, subcellular organelles and fractions, and nucleic acids from biological samples.
Chemical and medical use
Caesium fluoride enjoys a niche use in organic chemistry as a base and as an anhydrous source of fluoride ion
Nuclear and isotope applications
Caesium-137 is a radioisotope commonly used as a gamma-emitter in industrial applications. Its advantages include a half-life of roughly 30 years, its availability from the nuclear fuel cycle, and having 137Ba as a stable end product.
Cesium Physical Properties:
Appearance: | soft, gold-coloured metal |
Chemical Name: | Cesium metal |
Chemical Formula | Cs |
CAS No.: | 7440-46-2 |
Chemical Properties
ITEM | SPECIFICATION |
Cs | 99.9952% |
Li | 0.00018% |
Na | 0.002% |
K | 0.0015% |
Rb | 0.00008% |
Ca | 0.0010% |
Mg | 0.00009% |
Fe | 0.00016% |
Al | 0.00025% |
Pb | 0.00006% |
 |  |  |
-
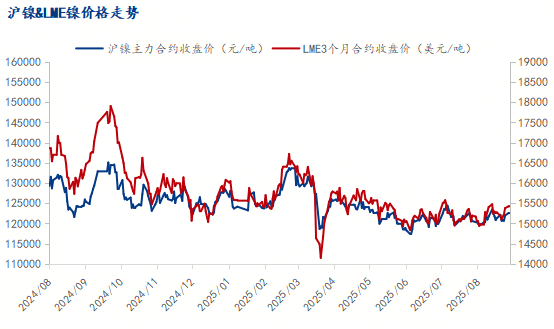
Nickel Market Update - October 28th

Oct,29,2025
-
Market Update on Nickel Prices in Nov.2025
Oct,29,2025
-

Oct,29,2025
-
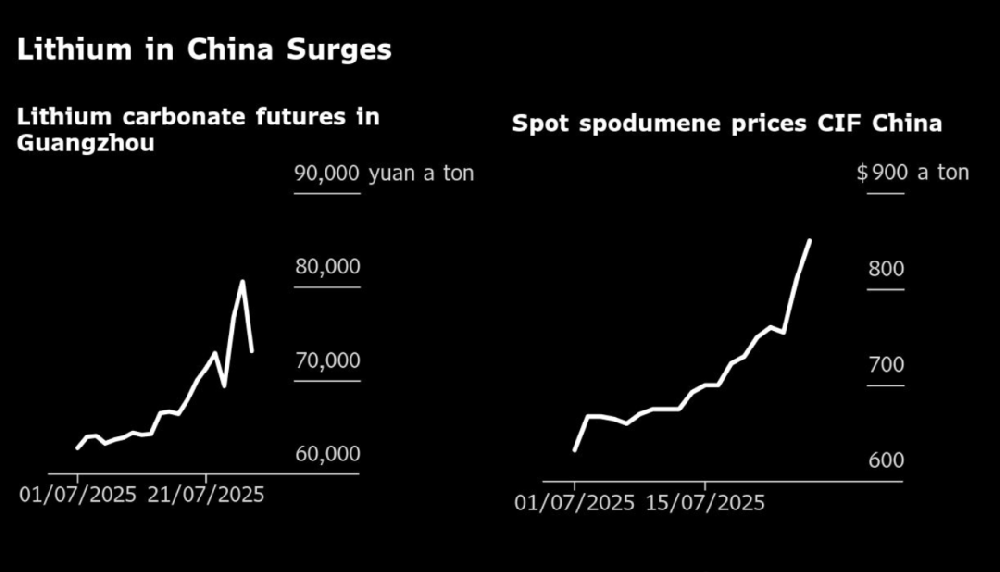
Mid week analysis of cobalt lithium new energy market and prices in October 2025
Oct,29,2025











