Understand the working principle of energy storage lithium batteries in one article
Jul,29,24
The development of energy storage technology has achieved remarkable results,
not only effectively meeting the needs of various stages of power grid operation, but also achieving peak shaving and valley filling, power load balancing,
and improving the acceptance of large-scale renewable energy in the power grid, as well as the possibility of intermittent renewable energy entering the grid.
Lithium battery technology is the most mature technology in electrochemical energy storage. Lithium batteries usually come in two shapes: cylindrical and rectangular.
The cylindrical battery adopts a spiral winding structure inside, which is separated between the positive and negative electrodes by a very fine and highly permeable thin film isolation material,
mainly including polyethylene, polypropylene, polyethylene and polypropylene composite materials.
The rectangular lithium battery is composed of stacked sheets, with a separator placed on the positive electrode, followed by the negative electrode, and so on, stacked one by one.
Composition of Energy Storage Lithium Battery
The main components of a lithium battery include a positive electrode, a negative electrode, an electrolyte, and a separator between the positive and negative electrodes.
The positive electrode includes a lithium-ion collection electrode composed of lithium containing materials (such as lithium cobalt oxide, lithium manganese oxide,
and a mixture of nickel cobalt manganese oxide), and a current collection electrode composed of an aluminum film.
The negative electrode is composed of a lithium-ion collector made of layered carbon material and a current collector made of copper thin film.
The battery is equipped with a safety valve and a Positive Temperature Coefficient (PTC) resistor,
which has the advantages of low thermal resistance, high heat transfer efficiency, non combustion, safety and reliability,
and can effectively prevent the battery from being damaged in abnormal states or output short circuits.
Diaphragm is a special composite module that prevents electrons from freely shuttling between the positive and negative electrodes in lithium batteries,
but lithium ions in the electrolyte can freely pass through.
Electrons cannot exist independently from the carrier, and the membrane is essentially an insulator that cannot contain free electrons, so it is non-conductive.
In batteries, elements exist mainly in the form of ions, which can easily pass through the separator, while electrons detach from their own elements onto a new carrier (positive or negative electrode material).
When in contact with the separator, the separator cannot absorb the free electrons on the electrode, thus preventing the passage of electrons.
The common diaphragm materials are single-layer PP film, PE film, and PP/PE/PP three-layer composite film.
Electrolytes are generally composed of lithium salts and organic solvents, which have the function of conducting ions.
Electrolytes facilitate the conduction of lithium ions between the positive and negative electrodes of batteries, with LiPF6 being the most widely used electrolyte currently.
Figure 1 provides an objective representation of the internal structure of the battery, which consists of lithium ions, metal ions, oxygen ions, and a carbon layer.
The composition of lithium batteries is mainly composed of compounds. The reaction of the battery is completed through the movement of ions inside the battery.
The separator of the battery acts as a barrier, separating the two poles of the battery.
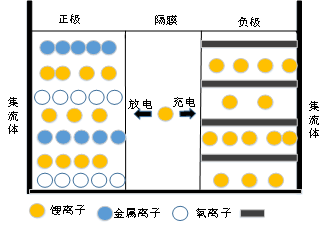
Figure 1 Schematic diagram of internal structure of lithium battery
Lithium batteries are currently indispensable portable energy storage components.
There are many external parameters that characterize the performance of lithium batteries, such as voltage, current, and internal resistance.
The advantages of lithium batteries are as follows:
1) The heat of combustion is very high, that is, the amount of heat released per unit volume is very high.
2) More environmentally friendly and in line with the requirements of green society development.
3) Long service life. Under normal conditions, lithium batteries can undergo hundreds of repeated charging and discharging processes, so they can be used for a long time.
4) No memory effect. If the battery frequently operates under fully charged and not fully discharged conditions, its capacity will quickly drop below the rated capacity value, a phenomenon called memory effect.
Ordinary batteries have a memory effect during operation, resulting in a decreasing capacity, which does not exist in lithium batteries.
Of course, lithium batteries have many other advantages, such as good safety performance, low self discharge rate, fast charging, and wide operating temperature range, so they are widely used.
Working principle of energy storage lithium battery
The internal chemical reaction of lithium batteries is a basic oxidation-reduction reaction, and energy is conserved.
Through chemical reactions, energy can be stored and used in the battery.
According to the chemical reaction equation,
the charging and discharging process of lithium batteries mainly relies on the difference in lithium electron concentration between the two poles,
which involves the insertion and extraction of lithium ions.
When charging a lithium battery, the lithium atoms in the positive electrode undergo an oxidation reaction, losing electrons and becoming lithium ions;
The large amount of lithium ions generated by the positive electrode oxidation reaction are deintercalated from the positive electrode,
pass through the electrolyte to reach the carbon layer of the negative electrode of the battery, and are embedded in the negative electrode.
At this point, the negative electrode of the lithium battery achieves a lithium rich state.
The size of battery capacity is related to both the number of lithium ions produced by the positive electrode reaction and the number of lithium ions exchanged through the electrolyte to the negative electrode.
Discharge is the reverse process of charging. During discharge, the negative electrode undergoes oxidation reaction, and the lithium ions embedded in the negative electrode carbon layer are deintercalated.
They are transported back to the positive electrode through the electrolyte, and the more lithium ions return to the positive electrode, the higher the discharge capacity.
When charging, lithium ions are generated at the positive electrode of the battery, and the generated lithium ions move through the electrolyte to the negative electrode.
The lithium ions at the negative electrode are embedded into the micropores of the carbon layer. The more lithium ions embedded, the higher the charging capacity.
The working principle of lithium batteries is shown in Figure 2.
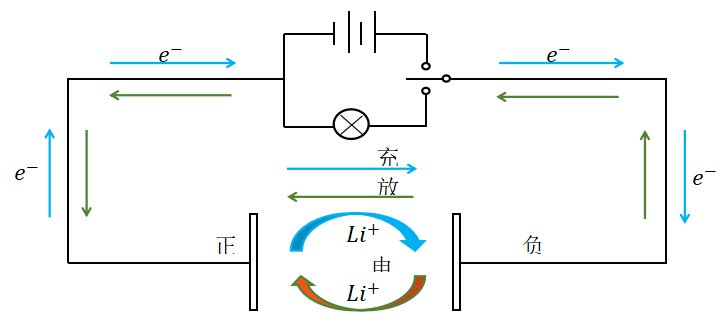
Figure 2 Working principle of lithium battery
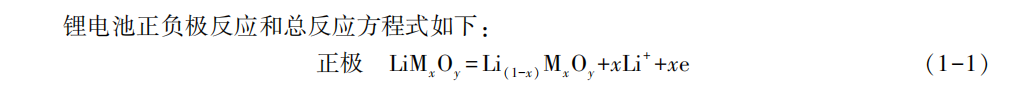
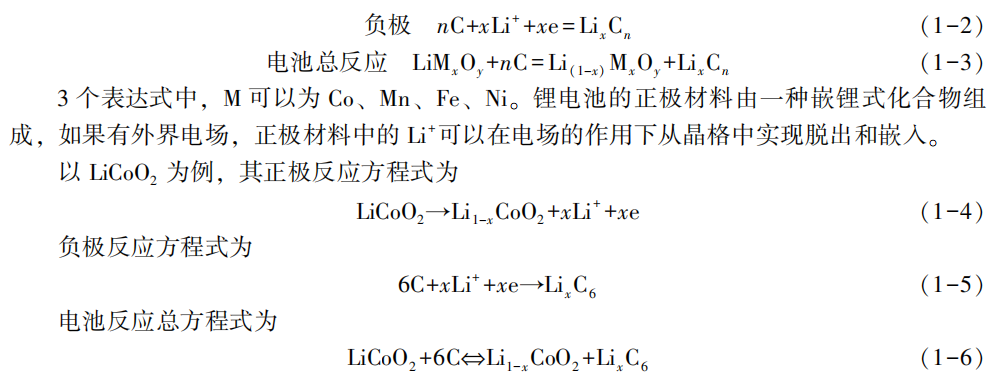
Segmented charging of energy storage lithium batteries
During use, lithium-ion batteries experience a decrease in voltage and chemical activity due to the release of electricity.
In order to better protect the functionality of lithium-ion batteries, a segmented charging strategy is adopted to ensure that the battery is quickly fully charged but not fully discharged,
which has a certain repairing effect on batteries that are not fully charged or discharged for a long time.
At present, there are two main charging methods for energy storage lithium batteries, namely constant current charging mode and constant voltage charging mode.
Whether it is constant current charging mode or constant voltage charging mode,
the charging method can be divided into four stages: trickle charging (low-voltage pre charging), constant current charging, constant voltage charging, and charging termination.
The charging of energy storage lithium batteries is mostly controlled by integrated circuit (IC) chips. The typical charging method is to first detect the actual voltage of the energy storage lithium battery to be charged.
If the voltage is lower than 3V, it needs to be pre charged first. At this time, the charging current is generally 1/10 of the set current until the voltage steadily rises to 3V before entering the standard charging process.
The standard charging process is: first, perform constant current charging with a set current, and then switch to constant voltage charging when the battery voltage rises to 4.2V,
keeping the charging voltage at 4.2V continuously for charging; After charging for a period of time, the charging current gradually decreases, and when it drops to 1/10 of the set current, the charging process ends.
The first stage: trickle charging. Trickle flow charging is mainly used for pre charging (restorative charging) of fully discharged battery cells.
When the battery voltage is below 3V, trickle charging is used.
The trickle charging current is 1/10 of the current under constant current charging mode,
which is 0.1C (C is a representation method of comparing the nominal capacity of the battery with the current, for example, if the battery capacity is 1000mA · h, then 1C represents a charging current of 1000mA).
Phase 2: Constant current charging. When the voltage value of the battery rises above the trickle charging threshold, increase the charging current at this time for constant current charging.
In general, the current value for constant current charging is between 0.2C and 1.0C. At this time, the voltage of the energy storage lithium battery will gradually increase with the constant current charging process.
Generally, the voltage set for a single battery is 3.0V~4.2V.
Phase 3: Constant voltage charging.
When the voltage of the energy storage lithium battery rises to 4.2V, the constant current charging phase ends and the constant voltage charging phase begins.
At this point, the change in current value will be determined by the saturation level of the battery cell.
As the charging process progresses, the charging current gradually decreases from its maximum value.
When it reaches 0.05C, it is considered that the charging is terminated.
Stage 4: Charging termination. There are two typical methods for determining the termination of charging: minimum charging current method and timing method (or a combination of both).
The first method uses the minimum current method to monitor the charging current during the constant voltage charging stage,
and terminates the charging when the charging current value decreases to 0.05C (or takes a value within the range of 0.02C~0.07C).
The second method sets the starting time from the constant voltage charging stage as the initial time, and terminates the charging process after continuous charging for 2 hours.






